- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics 复习笔记 5.3.6 Boyle's Law
Edexcel IGCSE Physics 复习笔记 5.3.6 Boyle's Law
Boyle's Law
- For a fixed mass of a gas held at a constant temperature:
pV = constant
- Where:
- p = pressure in pascals (Pa)
- V = volume in metres cubed (m3)
- This means that the pressure and volume are inversely proportional to each other
- When the volume decreases (compression), the pressure increases
- When the volume increases (expansion), the pressure decreases
- This is because when the volume decreases, the same number of particles collide with the walls of a container but more frequently as there is less space
- However, the particles still collide with the same amount of force meaning greater force per unit area (pressure)
- The key assumption is that the temperature and the mass (and number) of the particles remains the same
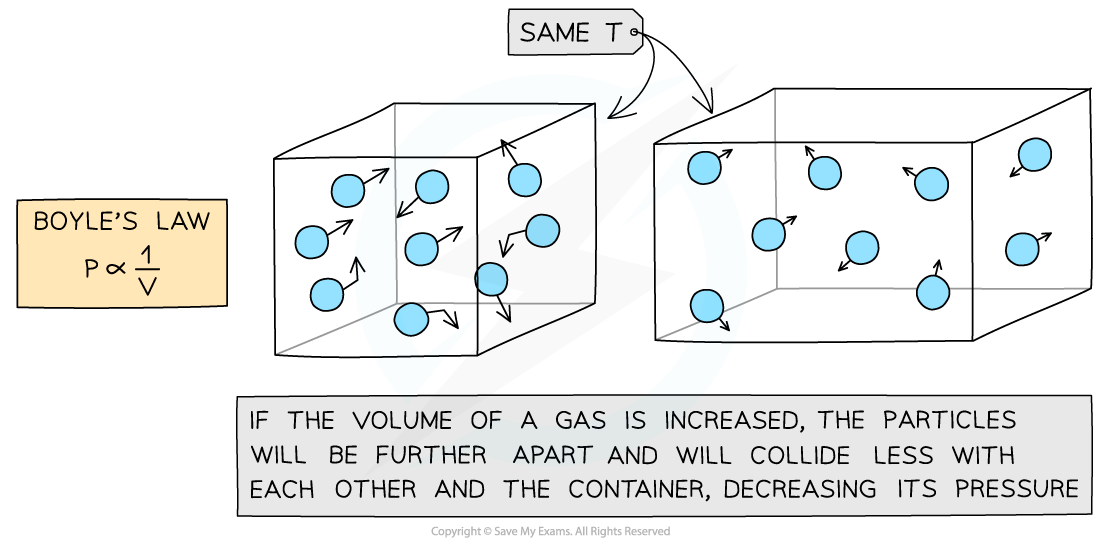
Increasing the volume of a gas decreases its pressure
- This equation can also be rewritten for comparing the pressure and volume before and after a change in a gas:
P1V1 = P2V2
- Where:
- P1 = initial pressure in pascals (Pa)
- V1 = initial volume in metres cubed (m3)
- P2 = final pressure in pascals (Pa)
- V2 = final volume in metres cubed (m3)
- This equation is sometimes referred to as Boyle's Law
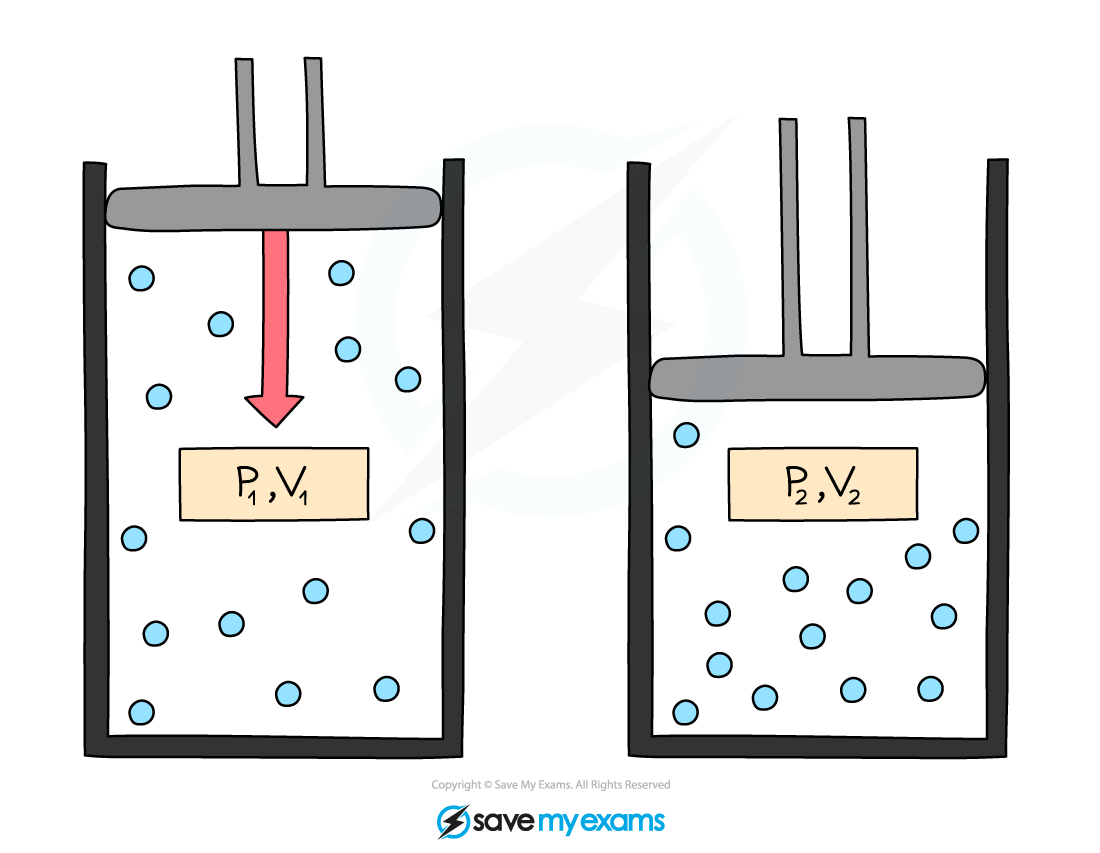
Initial pressure and volume, P1 and V1, and final pressure and volume, P2 and V2
Worked Example
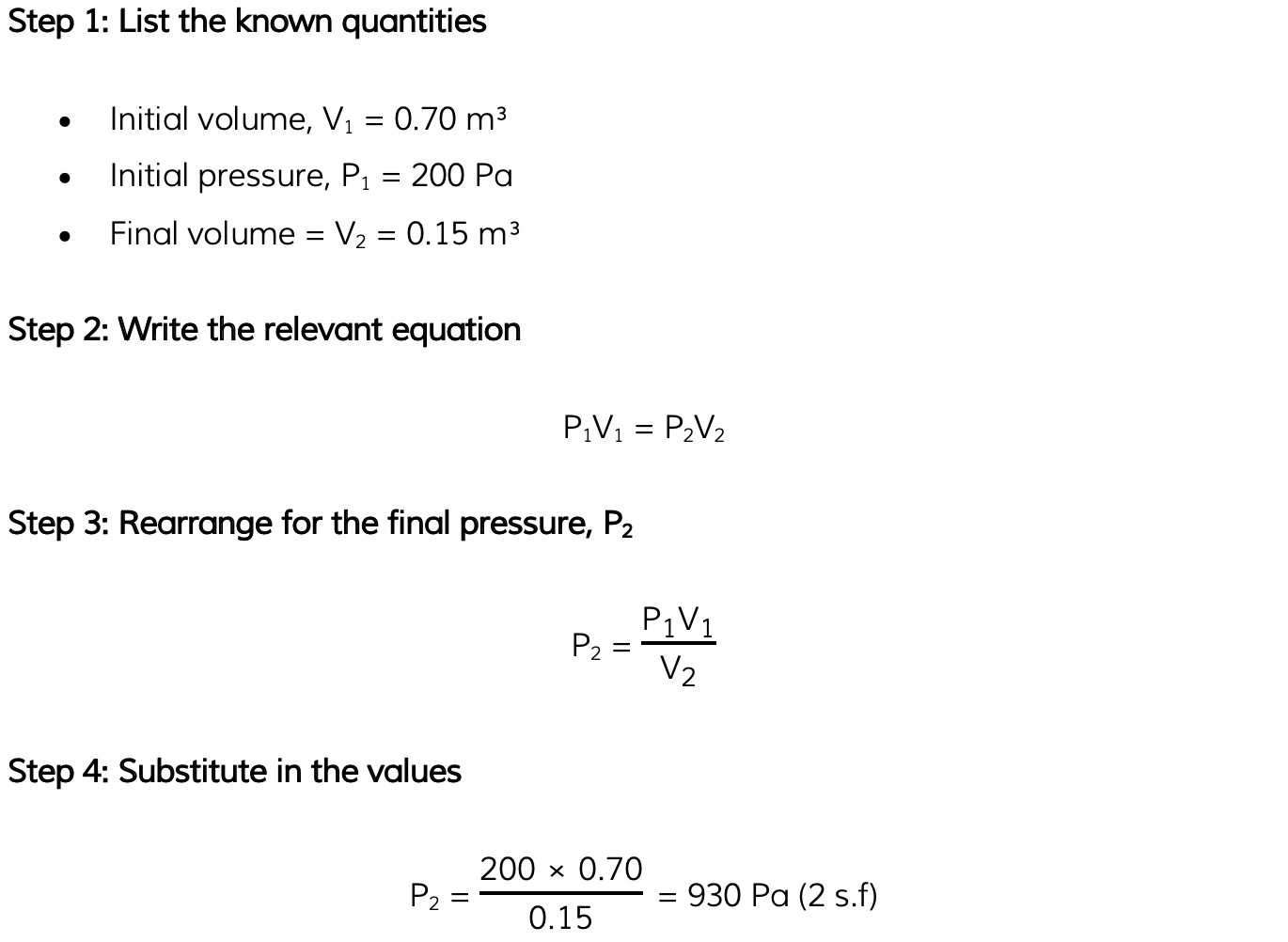
A gas occupies a volume of 0.70 m3 at a pressure of 200 Pa. Calculate the pressure exerted by the gas if it is compressed to a volume of 0.15 m3.Assume that the temperature and mass of the gas stay the same.
Exam Tip
Always check whether your final answer makes sense. If the gas has been compressed, the final pressure is expected to be more than the initial pressure (like in the worked example).If this is not the case, double-check the rearranging of any formulae and the values put into your calculator.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









