- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics 复习笔记 5.3.5 The Pressure Law
Edexcel IGCSE Physics 复习笔记 5.3.5 The Pressure Law
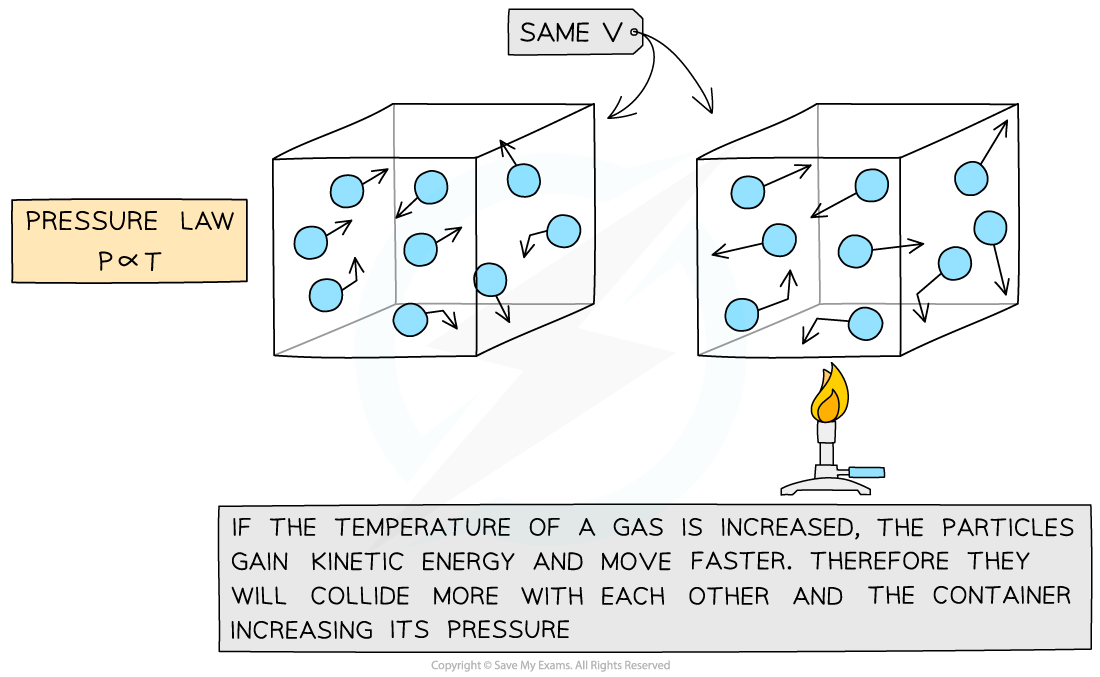
The Pressure Law
- If the volume V of an ideal gas is constant, the pressure law is given by:
P ∝ T
- This means the pressure is proportional to the temperature
- The relationship between the pressure and (Kelvin) temperature for a fixed mass of gas at constant volume can also be written as:
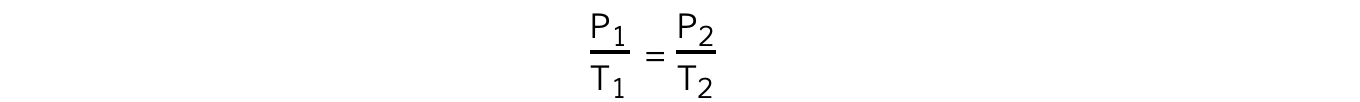
- Where:
- P1 = initial pressure (Pa)
- P2 = final pressure (Pa)
- T1 = initial temperature (K)
- T2 = final temperature (K)
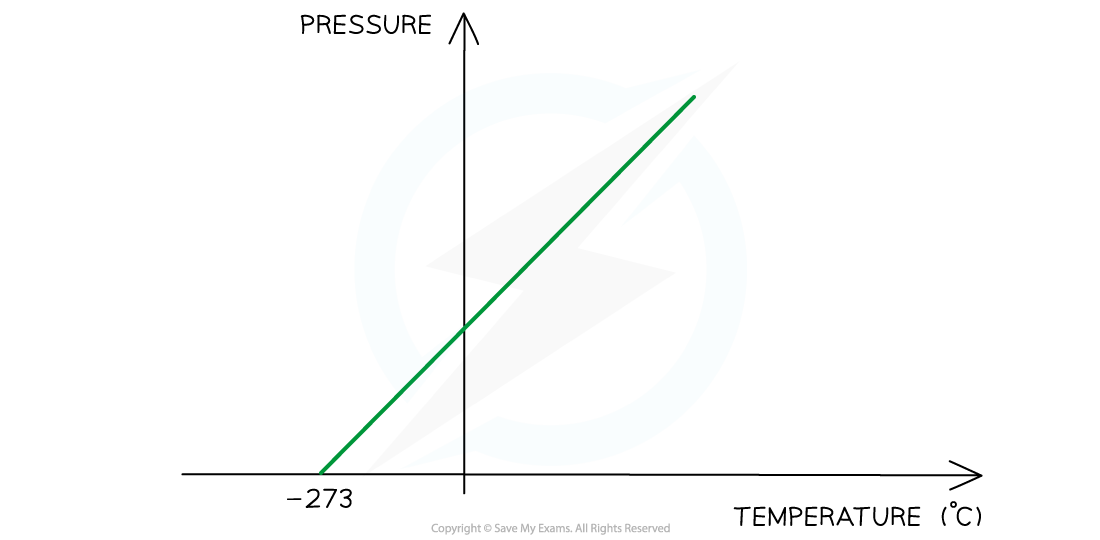
Pressure law graph representing temperature (in °C) directly proportional to the volume
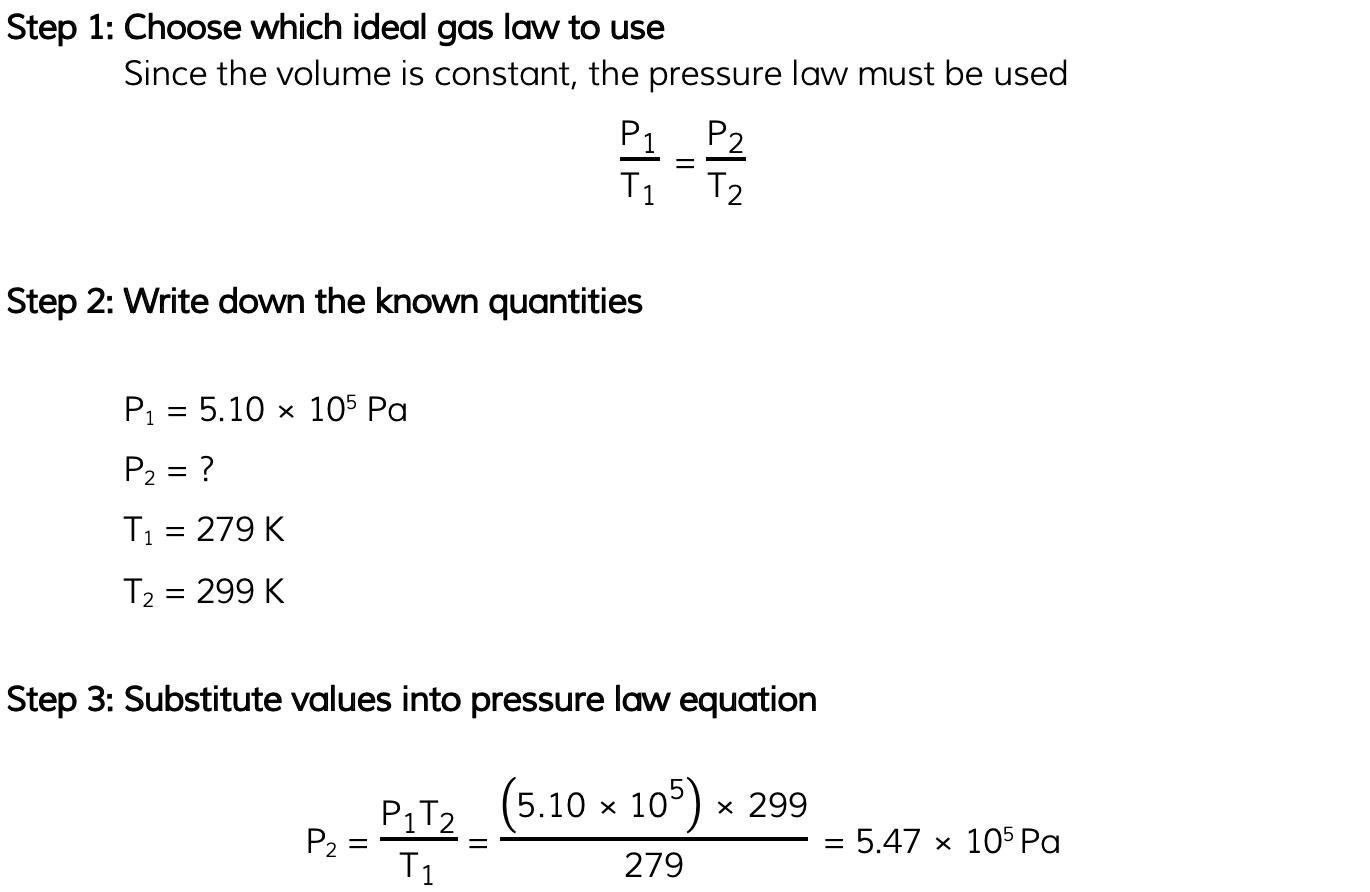
Worked Example
The pressure inside a bicycle tyre is 5.10 × 105 Pa when the temperature is 279 K. After the bicycle has been ridden, the temperature of the air in the tyre is 299 K.Calculate the new pressure in the tyre, assuming the volume is unchanged.
Exam Tip
Remember when using gas law the temperature T must always be in kelvin (K)!
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









