- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics 复习笔记 5.3.4 The Gas Laws
Edexcel IGCSE Physics 复习笔记 5.3.4 The Gas Laws
The Gas Laws
- Gas laws provide explanations for the relationships between:
- Pressure and volume at a constant temperature
- Pressure and (Kelvin) temperature at a constant volume
Pressure & Volume
- If the temperature of a gas remains constant, the pressure of the gas changes when it is:
- Compressed – decreases the volume which increases the pressure
- Expanded – increases the volume which decreases the pressure
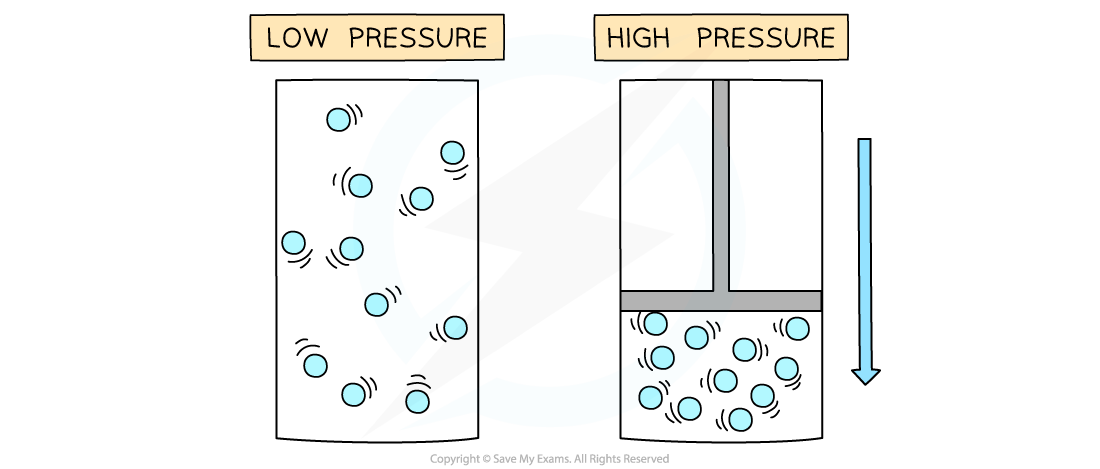
Pressure increases when a gas is compressed
- Similarly, a change in pressure can cause a change in volume
- A vacuum pump can be used to remove the air from a sealed container
- The diagram below shows the change in volume to a tied up balloon when the pressure of the air around it decreases:
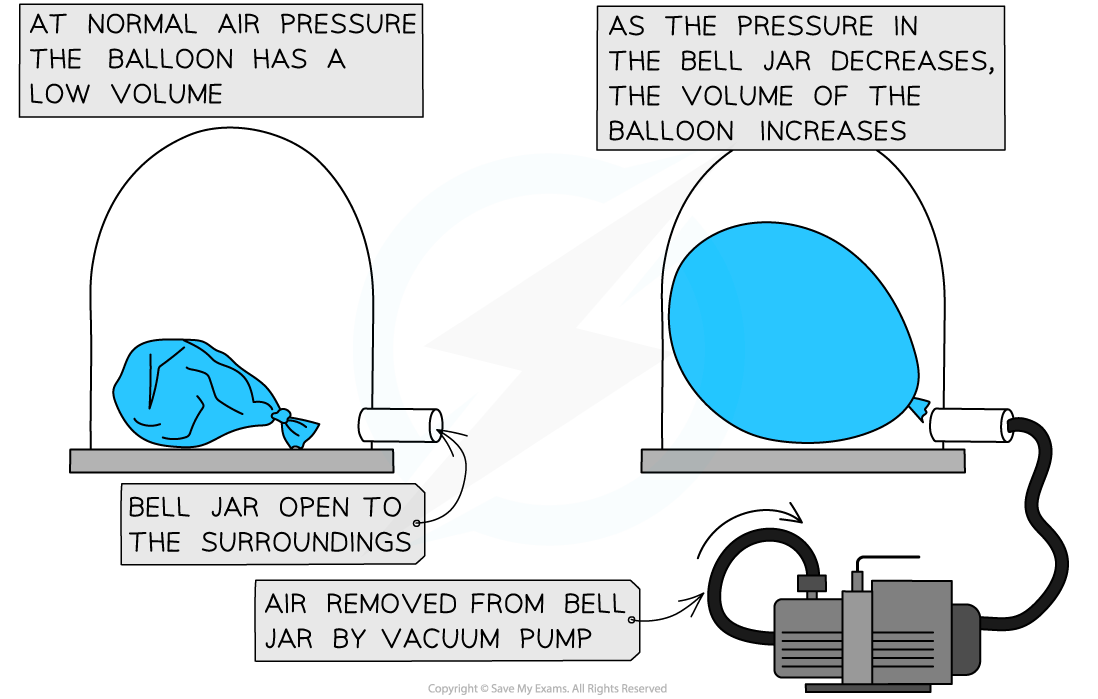
- Therefore, if the gas is compressed, the molecules will hit the walls of the container more frequently
- This creates a larger overall net force on the walls which increases the pressure
Pressure & Temperature
- The motion of molecules in a gas changes according to the temperature
- As the temperature of a gas increases, the average speed of the molecules also increases
- Since the average kinetic energy depends on their speed, the kinetic energy of the molecules also increases if its volume remains constant
- The hotter the gas, the higher the average kinetic energy
- The cooler the gas, the lower the average kinetic energy
- If the gas is heated up, the molecules will travel at a higher speed
- This means they will collide with the walls more often
- This creates an increase in pressure
- Therefore, at a constant volume, an increase in temperature increases the pressure of a gas and vice versa
- Diagram A shows molecules in the same volume collide with the walls of the container more with an increase in temperature
- Diagram B shows that since the temperature is proportional to the pressure, the graph against each is a straight line
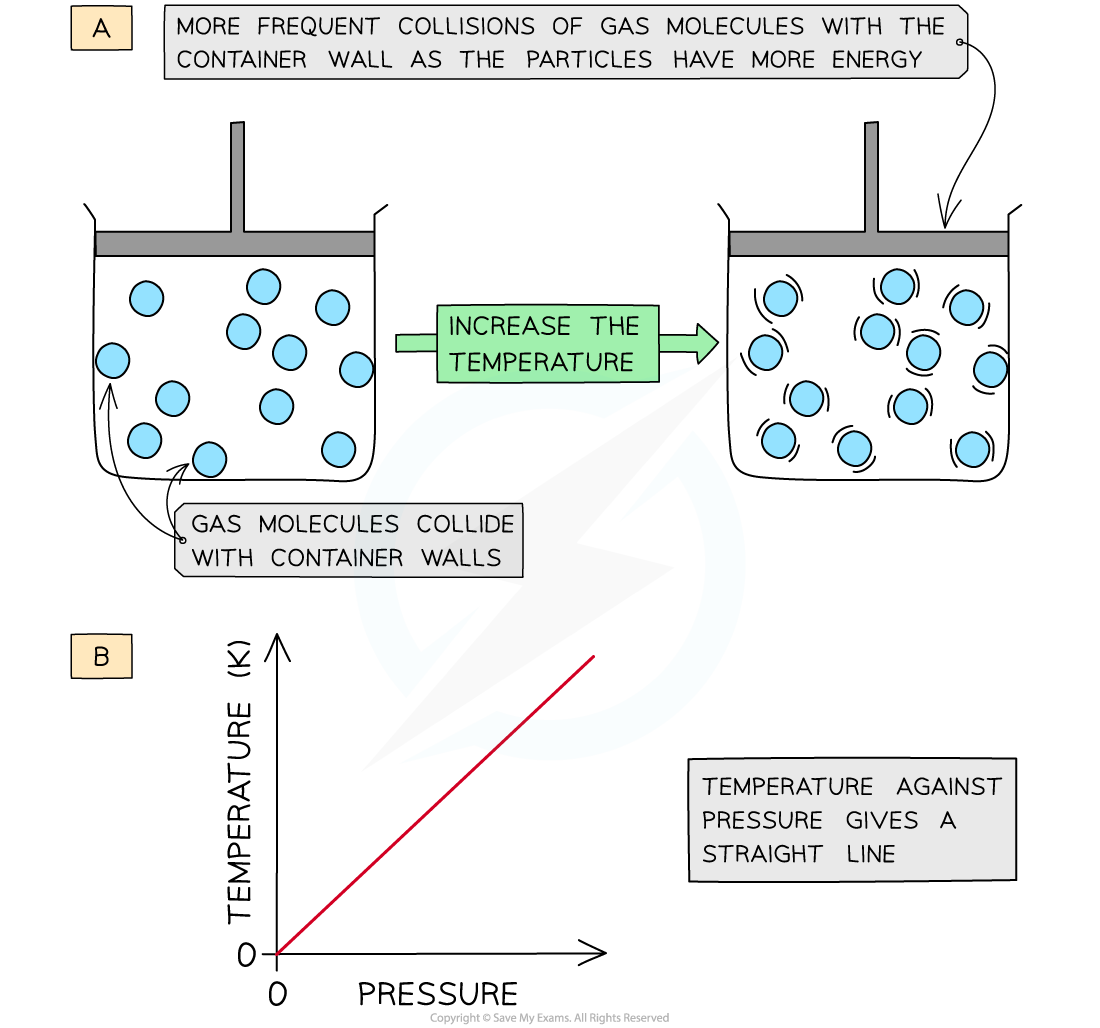
At constant volume, an increase in the temperature of the gas increases the pressure due to more collisions on the container walls
Exam Tip
You are required to be able to describe the links between pressure & volume and pressure & temperature qualitatively. This means that the correct use of terms such as 'collision', 'kinetic energy' and 'frequency', will be really important.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








