- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics 复习笔记 5.3.2 Absolute Zero
Edexcel IGCSE Physics 复习笔记 5.3.2 Absolute Zero
Absolute Zero
- The amount of pressure that a gas exerts on its container is dependent on the temperature of the gas
- This is because particles move with more energy as their temperature increases
- As the temperature of the gas decreases, the pressure on the container also decreases
- In 1848, Mathematician and Physicist, Lord Kelvin, recognised that there must be a temperature at which the particles in a gas exert no pressure
- At this temperature they must no longer be moving, and hence not colliding with their container
- This temperature is called absolute zero and is equal to -273 °C
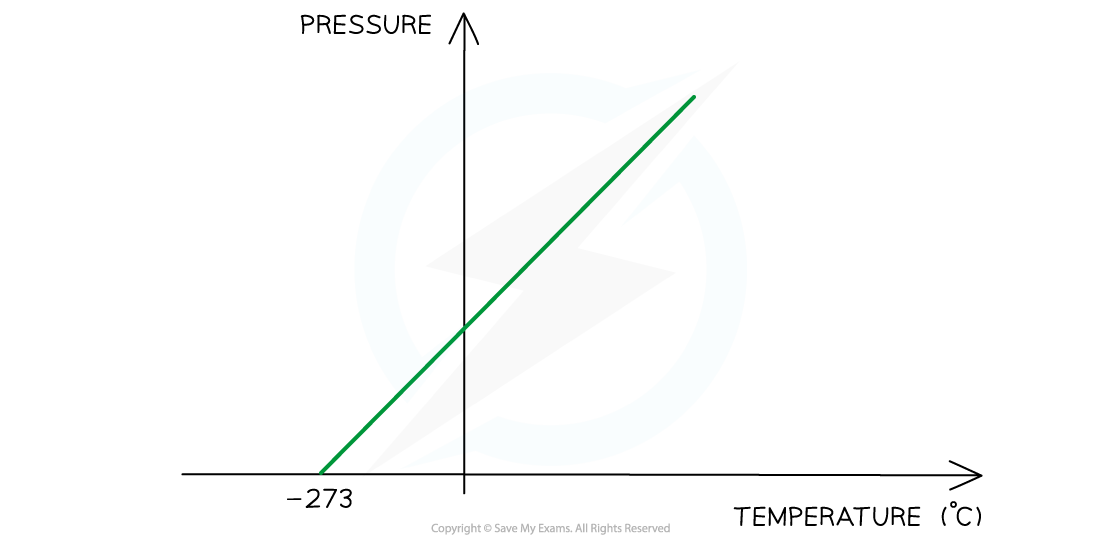
At absolute zero, or -273 °C, particles will have no net movement. It is therefore not possible to have a lower temperature
- Absolute zero is defined as:
The temperature at which the molecules in a substance have zero kinetic energy
- This means for a system at absolute zero, it is not possible to remove any more energy from it
- Even in space, the temperature is roughly 2.7 K above absolute zero
The Kelvin Scale
- The Kelvin temperature scale begins at absolute zero
- 0 K is equal to -273 °C
- An increase of 1 K is the same change as an increase of 1 °C
- It is not possible to have a temperature lower than 0 K
- This means a temperature in Kelvin will never be a negative value
- To convert between temperatures θ in the Celsius scale, and T in the Kelvin scale, use the following conversion:
θ / °C = T / K − 273
T / K = θ / °C + 273
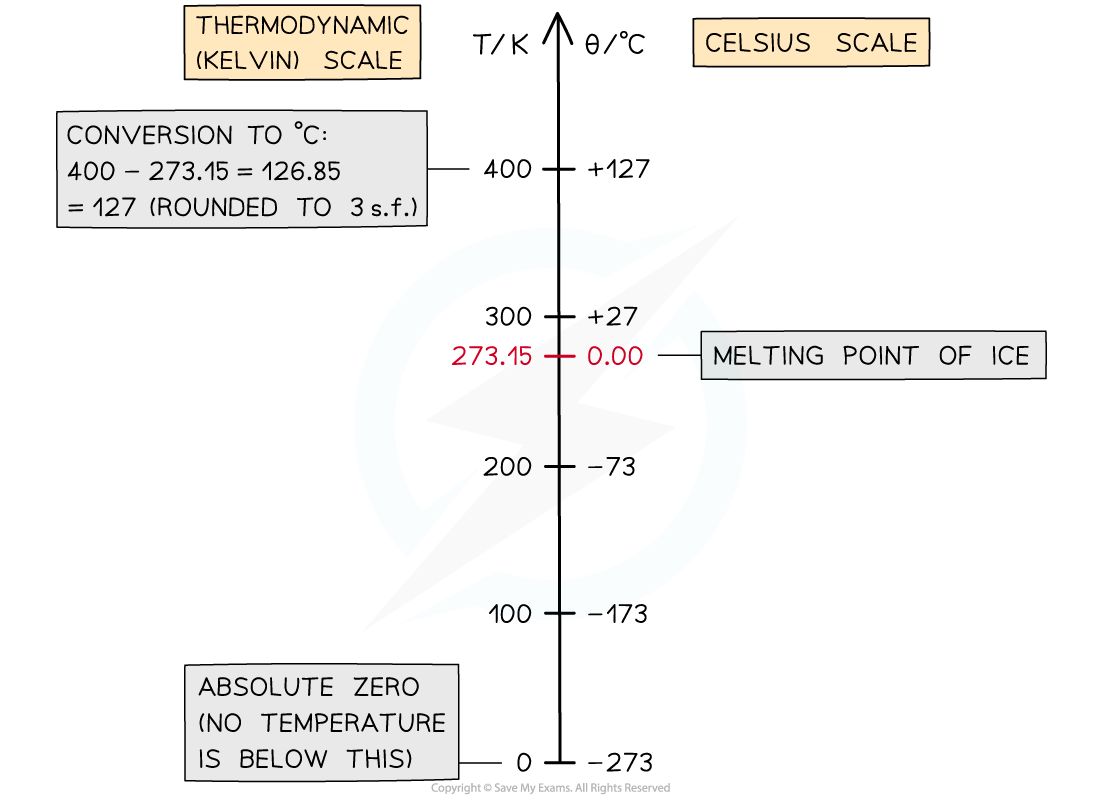
Conversion chart relating the temperature on the Kelvin and Celsius scales
- The divisions on both scales are equal. This means:
A change in a temperature of 1 K is equal to a change in temperature of 1 °C
Worked Example
The temperature in a room is 300 K.What is this temperature in Celsius?
Step 1: Kelvin to Celsius equation
θ / °C = T / K − 273
Step 2: Substitute in value of 300 K
300 K − 273 = 27 °C
Exam Tip
If you forget in the exam whether it’s +273 or −273, just remember that 0 °C = 273 K. This way, when you know that you need to +273 to a temperature in degrees to get a temperature in Kelvin. For example: 0 °C + 273 = 273 K.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








