- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics 复习笔记 5.2.4 Specific Heat Capacity
Edexcel IGCSE Physics 复习笔记 5.2.4 Specific Heat Capacity
Specific Heat Capacity
- How much the temperature of a system increases depends on:
- The mass of the substance heated
- The type of material
- The amount of energy put in to the system
- The energy put in is in the form of thermal energy
- The specific heat capacity, c of a substance is defined as:
The amount of energy required to raise the temperature of 1 kg of the substance by 1 °C
- Different substances have different specific heat capacities
- If a substance has a low specific heat capacity, it heats up and cools down quickly (ie. it takes less energy to change its temperature)
- If a substance has a high specific heat capacity, it heats up and cools down slowly (ie. it takes more energy to change its temperature)
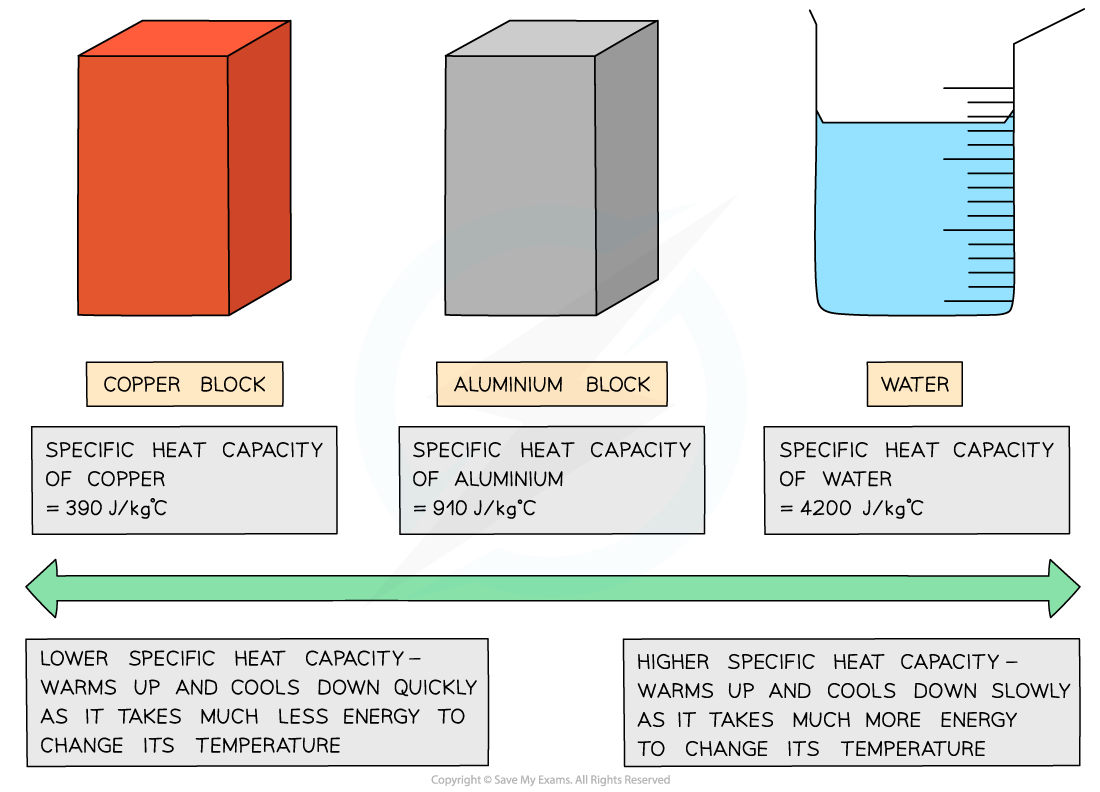
Low vs high specific heat capacity
Calculating Specific Heat Capacity
- The amount of energy needed to raise the temperature of a given mass by a given amount can be calculated using the equation:
Change in thermal energy = Mass × Specific heat capacity × Change in temperature
ΔQ = mcΔT
- Where:
- ΔQ = change in thermal energy, in joules (J)
- m = mass, in kilograms (kg)
- c = specific heat capacity, in joules per kilogram per degree Celsius (J/kg °C)
- ΔT = change in temperature, in degrees Celsius (°C)
Worked Example
Water of mass 0.48 kg is increased in temperature by 0.7 °C. The specific heat capacity of water is 4200 J / kg °C.Calculate the amount of thermal energy transferred to the water.
Step 1: Write down the known quantities
-
- Mass, m = 0.48 kg
- Change in temperature, ΔT = 0.7 °C
- Specific heat capacity, c = 4200 J/kg °C
Step 2: Write down the relevant equation
ΔQ = mcΔT
Step 3: Calculate the thermal energy transferred by substituting in the values
ΔQ = (0.48) × (4200) × (0.7) = 1411.2
Step 4: Round the answer to 2 significant figures
ΔQ = 1400 J
Exam Tip
This equation will be given on your equation sheet, so don't worry if you cannot remember it, but it is important that you understand how to use it. You will always be given the specific heat capacity of a substance, so you do not need to memorise any values.
转载自savemyexam

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1