- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Physics 复习笔记 5.2.2 Changes of State
Edexcel IGCSE Physics 复习笔记 5.2.2 Changes of State
Changes of State
- When a substance changes state, the number of molecules in that substance doesn’t change and so neither does its mass
- The only thing that changes is its energy
- Unlike chemical changes, changes of state (a type of physical change) are reversible
- In a solid:
- The molecules are very close together and arranged in a regular pattern
- The molecules vibrate about fixed positions
- In a liquid:
- The molecules are still close together (no gaps) but are no longer arranged in a regular pattern
- The molecules are able to slide past each other
- In a gas:
- The molecules are widely separated - about 10 times further apart in each direction
- The molecules move about randomly at high speeds
- There are six changes of state that can occur between solids, liquids and gases:
- Melting - When a solid turns into a liquid (e.g. ice to water)
- Boiling - When a liquid turns into a gas (evaporating)
- Condensing - When a gas turns into a liquid
- Freezing - When a liquid turns into a solid
- Subliming -When a solid turns into a gas
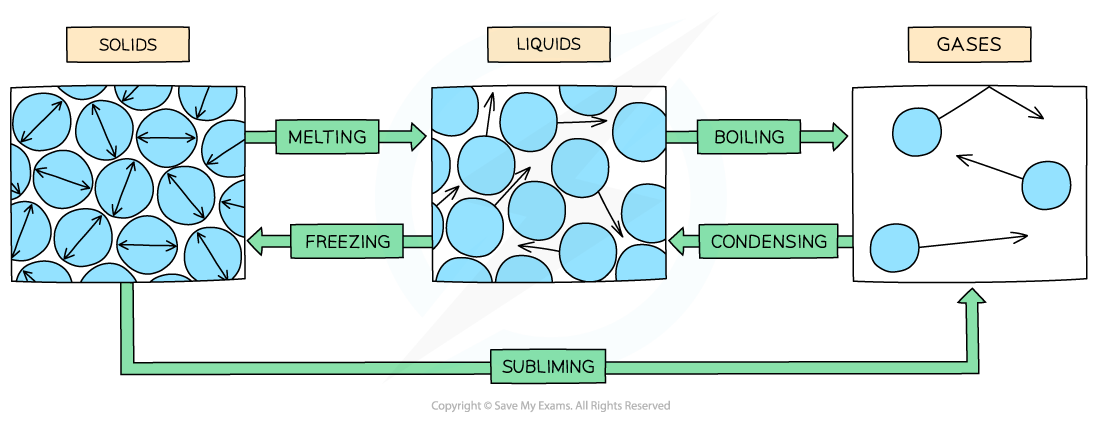
Diagram showing the arrangement and motion of different states of matter
Heat & Temperature
- Heating a system will change the energy stored in a system by increasing the kinetic energy of its particles
- The temperature of the material, therefore, is related to the average kinetic energy of the molecules
- This increase in kinetic energy (and therefore energy stored in the system) can:
- Cause the temperature of the system to increase
- Or, produce a change of state (solid to liquid or liquid to gas)
- The higher the temperature, the higher the average kinetic energy of the molecules and vice versa
- This means they move around faster
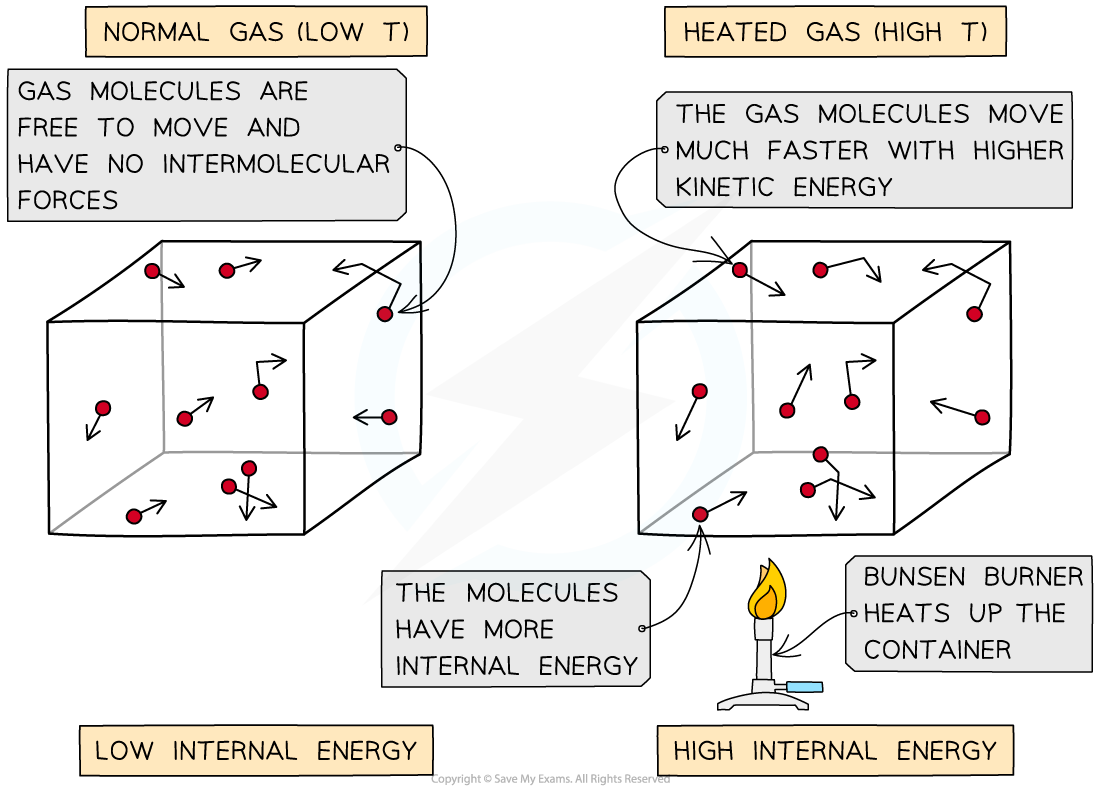
As the container is heated up, the gas molecules move faster with higher kinetic energy. The energy stored within the system - the internal energy - therefore increases
Worked Example
A student measures the mass of a beaker of water twice, leaving 24 hours between the readings. The temperature in the room remained constant between readings, however, they notice a decrease in the mass of the beaker of water.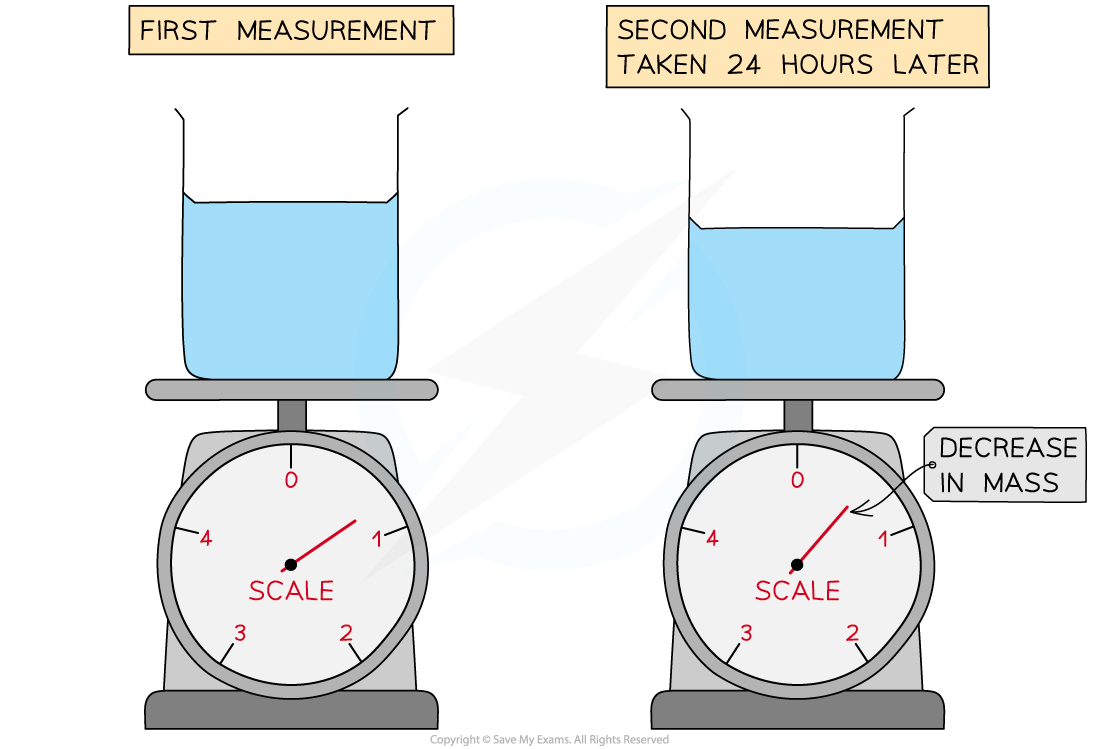 Which of the following is not a correct conclusion that can be drawn from the experiment?
Which of the following is not a correct conclusion that can be drawn from the experiment?
A The difference in mass is equal to the mass of the water that evaporated
B The total energy within the beaker decreased
C The density of water in the air increased
D The total number of water molecules in the air and water decreased
ANSWER: D
-
- A is true because the mass lost from the beaker is due to those water molecules evaporating
- B is true because evaporation causes the most energetic particles to leave the beaker
- The total number of particles in the beaker decreased
- C is true because additional water molecules were added to the air, without a significant change in the volume of the air
- D is not true because no mass is lost during evaporation - it is only changed from a liquid to gas state
Exam Tip
Heating a system will always increase the energy stored within the system.You should remember that this increase in 'internal energy' can have two effects: either the temperature of the system will increase, or the system will change state (e.g. from a solid to a liquid, or a liquid to a gas)
转载自savemyexam

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









