- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 4.5.2 Oxidation of Ethanol
Edexcel IGCSE Chemistry 复习笔记 4.5.2 Oxidation of Ethanol
Oxidation of Ethanol
- Ethanol can undergo oxidation in three different ways:
- Combustion
- Aerobic oxidation
- Treatment with an oxidising agent
Combustion
- Alcohols undergo combustion to form carbon dioxide and water
- The complete combustion of ethanol is as follows:
- Ethanol burns readily with an almost invisible blue flame
- School laboratories use ethanol in spirit burners as it burns cleanly and without strong odours
Aerobic Oxidation
- Bacteria in the air (acetobacter) use atmospheric oxygen from air to oxidise the ethanol in the wine:
ethanol + oxygen → ethanoic acid + water
- The acidic, vinegary taste of wine which has been left open for several days is due to the presence of ethanoic acid
- This is what happens to wine when it is left open as the microbial oxidation of ethanol will produce a weak solution of the carboxylic acid, ethanoic acid, the same acid used in vinegar
Treatment with an oxidising agent
- Alcohols undergo oxidation to produce carboxylic acids when treated with oxidising agents
- When ethanol is heated with acidified potassium dichromate solution the ethanol oxidises to ethanoic acid
- The equation for the reaction is:
CH3CH2OH + [O] → CH3COOH + H2O
- The oxidising agent is represented by the symbol for oxygen in square brackets
- The reaction is slow so the mixture is heated to its boiling point for about an hour; to avoid the substances evaporating a condenser is placed above the reaction flask that prevents volatile liquids from escaping
- During the reaction the potassium dichromate turns from orange to green
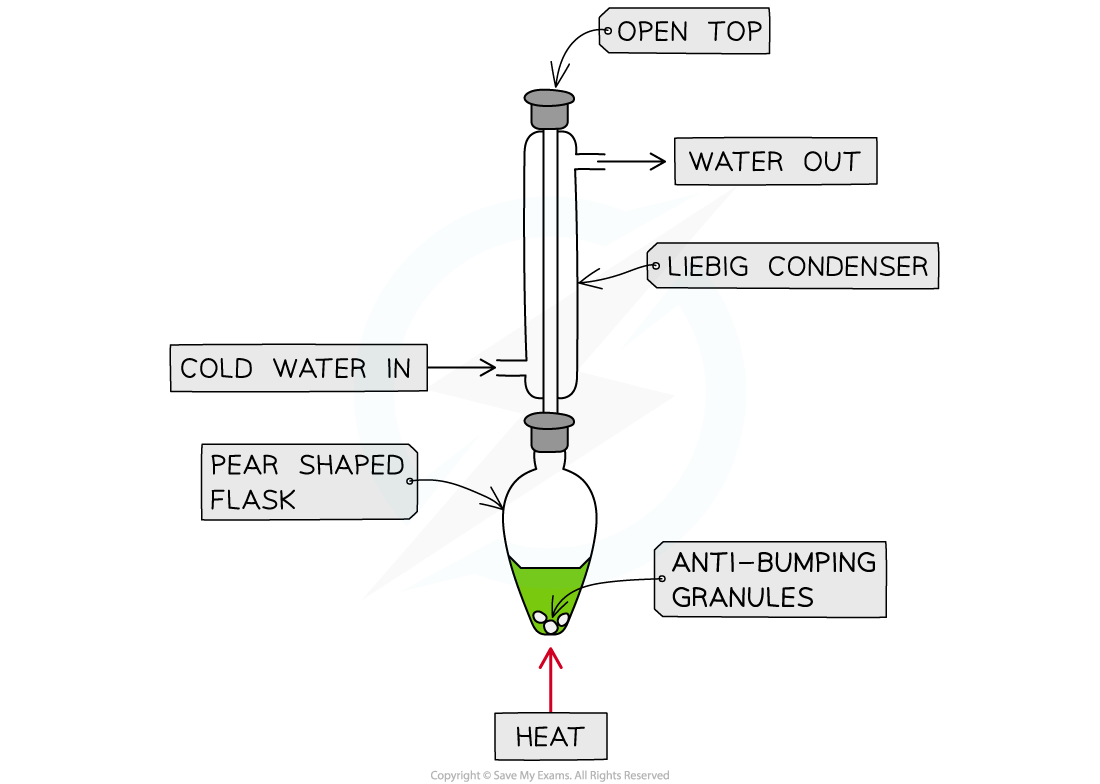
Ethanol can be oxidised by heating it with potassium dichromate in sulfuric acid. The solution turns from orange to green during the reaction
Exam Tip
Be careful when writing the equation for the combustion of ethanol- students often forget to include the oxygen in the ethanol when balancing the equation.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1










