- The empirical formula shows the simplest possible ratio of the atoms in a molecule
- For example: Hydrogen peroxide is H2O2 but the empirical formula is HO
- The molecular formula shows the actual number of atoms in a molecule
- For example:
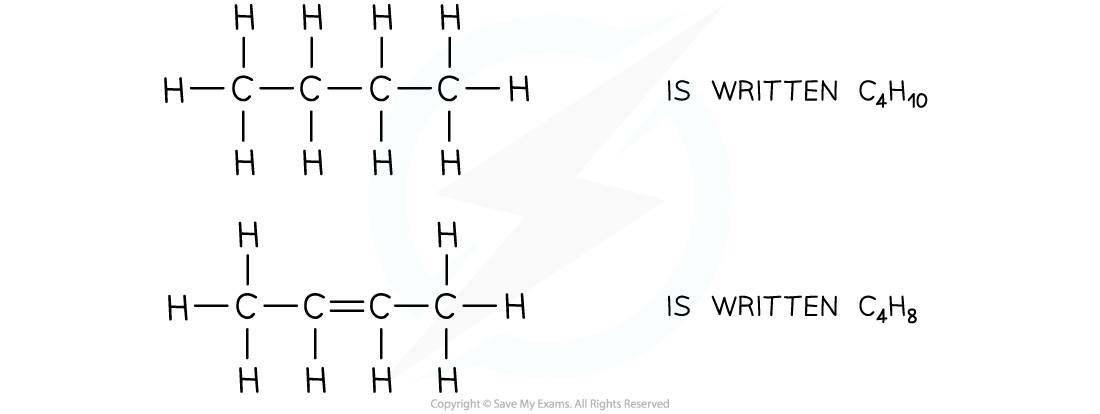
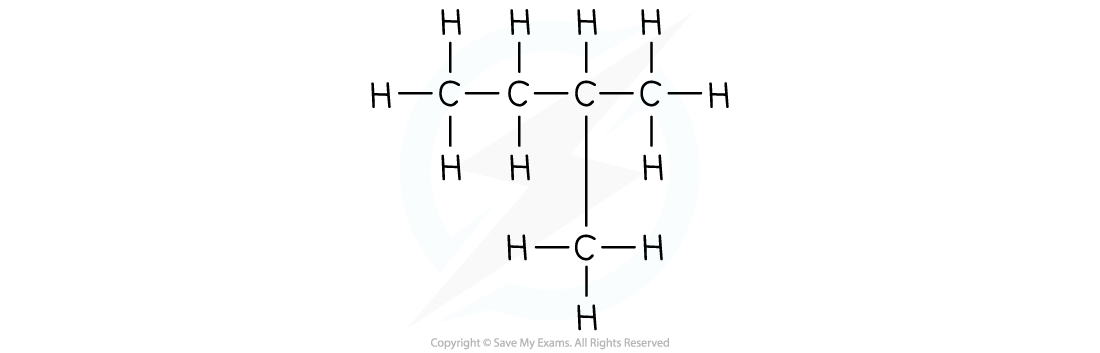

For defining a hydrocarbon, you must specify that they are compounds which contain hydrogen and carbon atoms only, no other element is present.You may not be asked to name branched chain organic compounds but you will come across them. It is useful to know that the numbers in the names of these compounds refer to the position of the side chains with respect to the main chain.
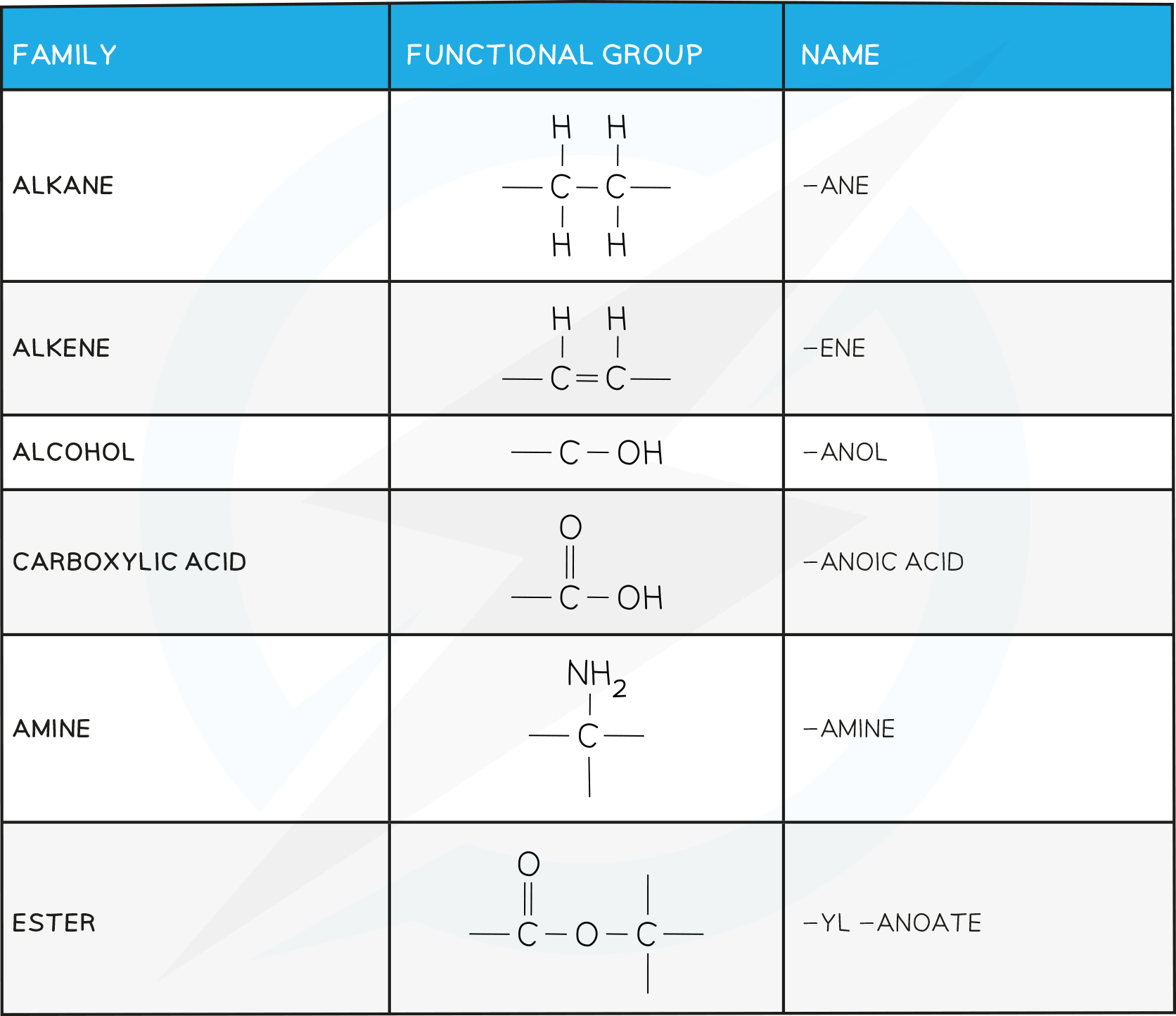
Structures and Names of Common Functional Groups
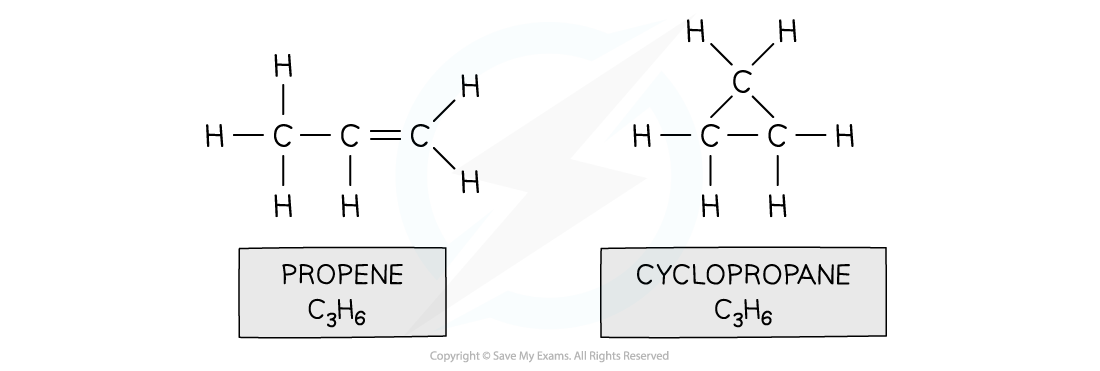
Isomers of C3H6 show the same molecular formula but different structures. Isomers can show similar physical and chemical properties or if they have different functional groups, the properties can be different.
转载自savemyexams

© 2025. All Rights Reserved. 沪ICP备2023009024号-1