Practical: Effect of Catalysts on Rate of Reaction
Aim:
To investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide
Diagram:
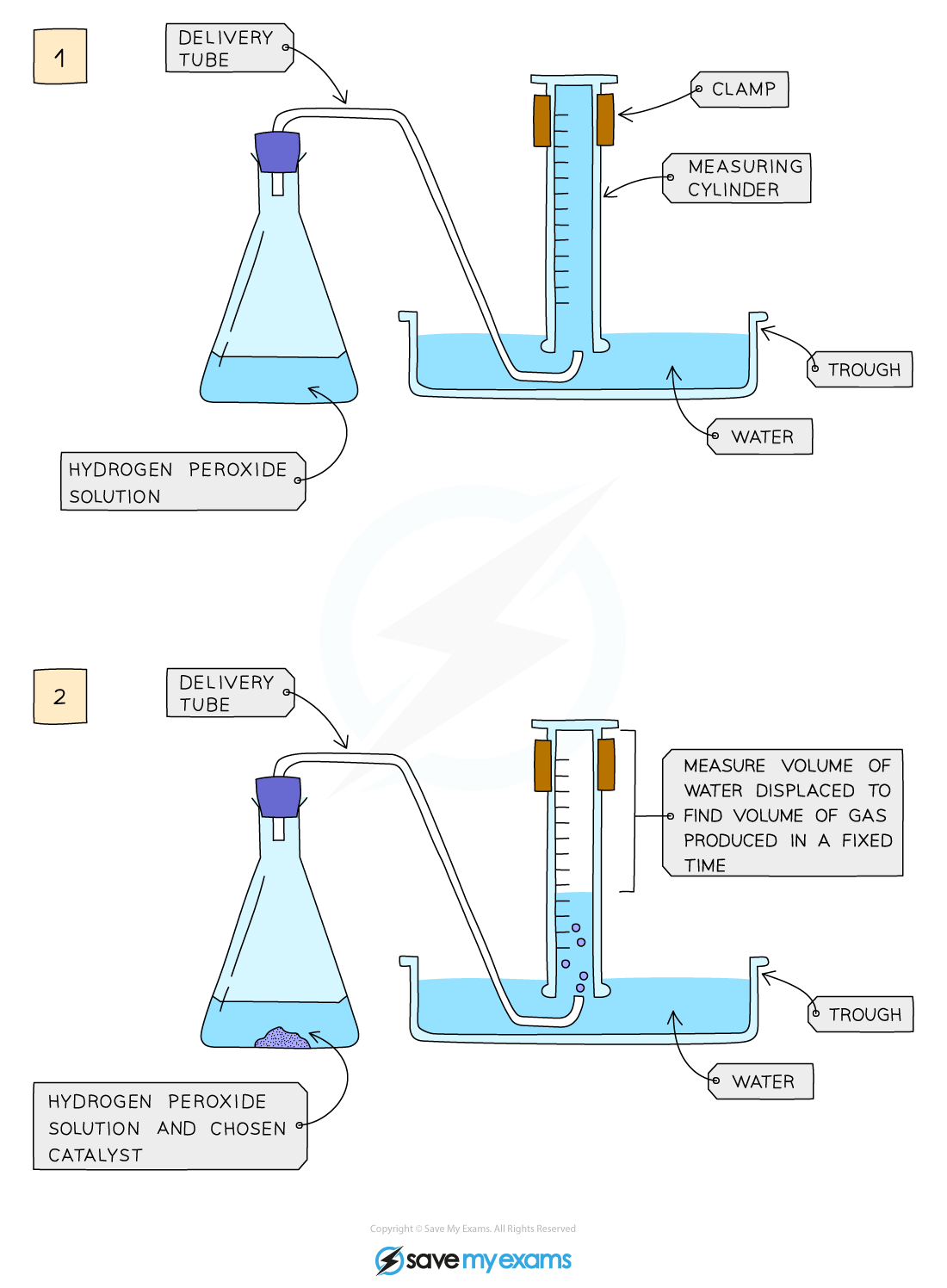
Diagram showing the apparatus needed to investigate the effect of a catalyst on the rate of reaction
Method:
- Add hydrogen peroxide into a conical flask
- Use a delivery tube to connect this flask to a measuring cylinder upside down in water trough
- Add the chosen catalyst into the conical flask and close the bung
- Measure the volume of gas produced in a fixed time using the measuring cylinder
- Repeat experiment with different catalysts and compare results
- Catalysts to try could include: manganese(IV) oxide, lead(II) oxide, iron(III) oxide and copper(II) oxide
Result:
- The data for different catalysts can be plotted on the same graph and the relative rates compared
转载自savemyexams










