Practical: Effect of Surface Area on Rate of Reaction
Aim:
To investigate the effect of changing surface area on a reaction rate
Diagram:
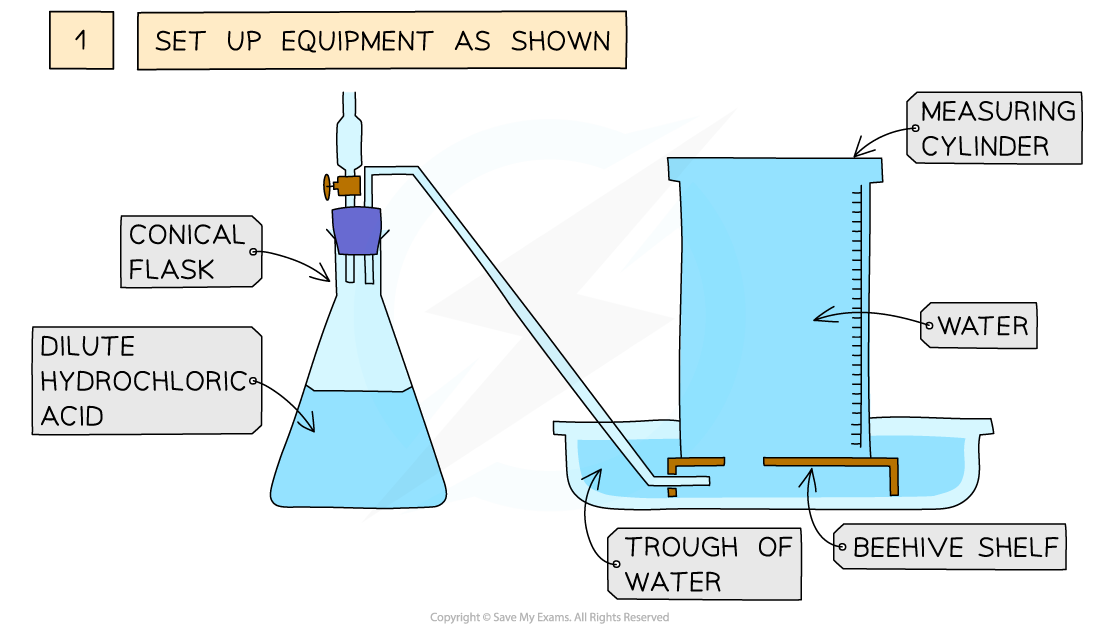
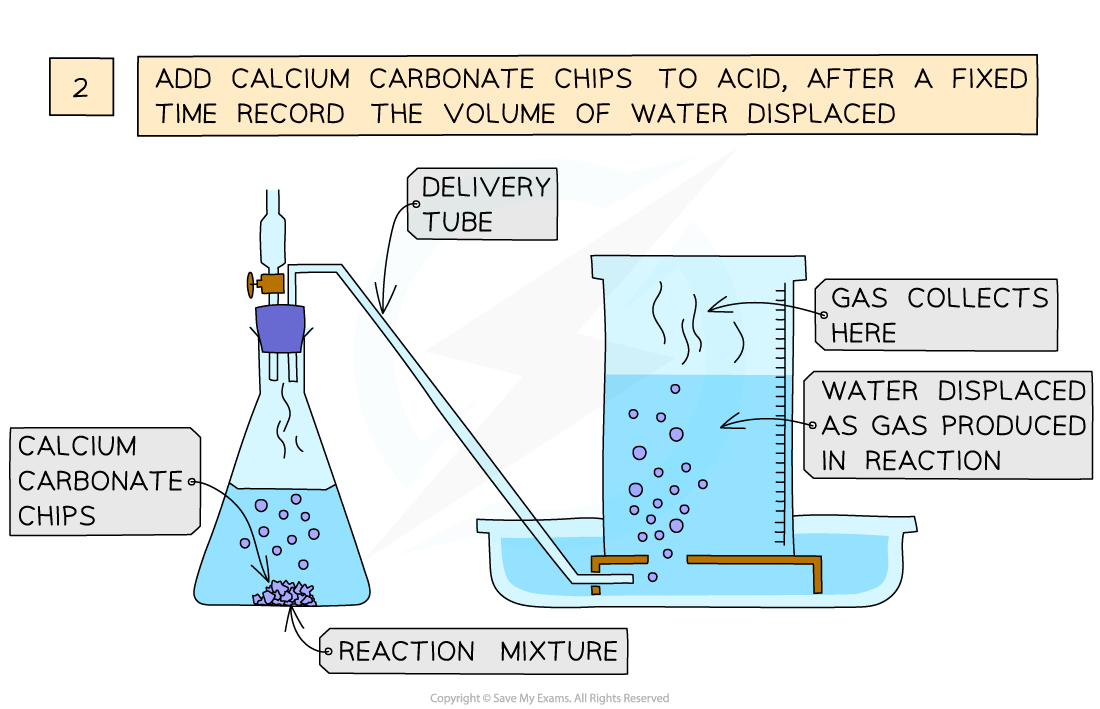
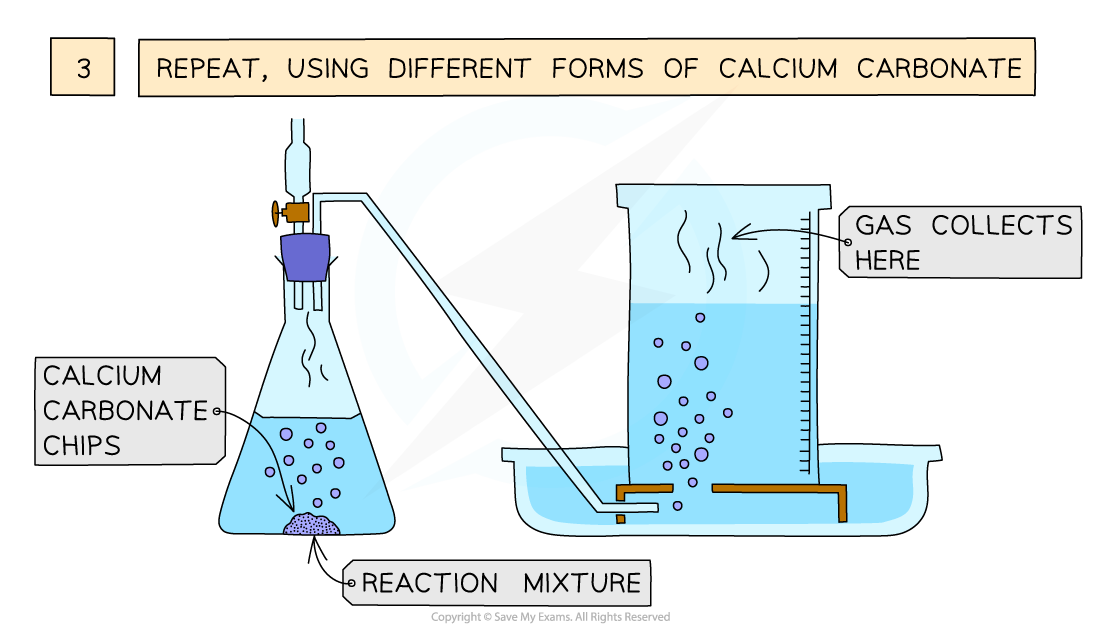
Investigating the effect of different size marble chips on the rate of reaction between calcium carbonate and hydrochloric acid
Method:
- Add hydrochloric acid into a conical flask
- Use a delivery tube to connect this flask to an inverted measuring cylinder
- Add marble chips into the conical flask and close the bung
- Measure the volume of gas produced in a fixed time using the measuring cylinder
- Repeat with different sizes of marble chips / concentrations of hydrochloric acid
Result:
- Increase in the surface area of the marble chip, the rate of reaction will increase
- This is because more surface area particles of the marble chips will be exposed to the dilute hydrochloric acid so there will be more frequent and successful collisions, increasing the rate of reaction
转载自savemyexams










