- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 2.7.9 Practical: Prepare Lead(II)Sulfate
Edexcel IGCSE Chemistry 复习笔记 2.7.9 Practical: Prepare Lead(II)Sulfate
Practical: Prepare Lead(II)Sulfate
Aim:
To prepare a dry sample of lead(II) sulfate
Diagram:
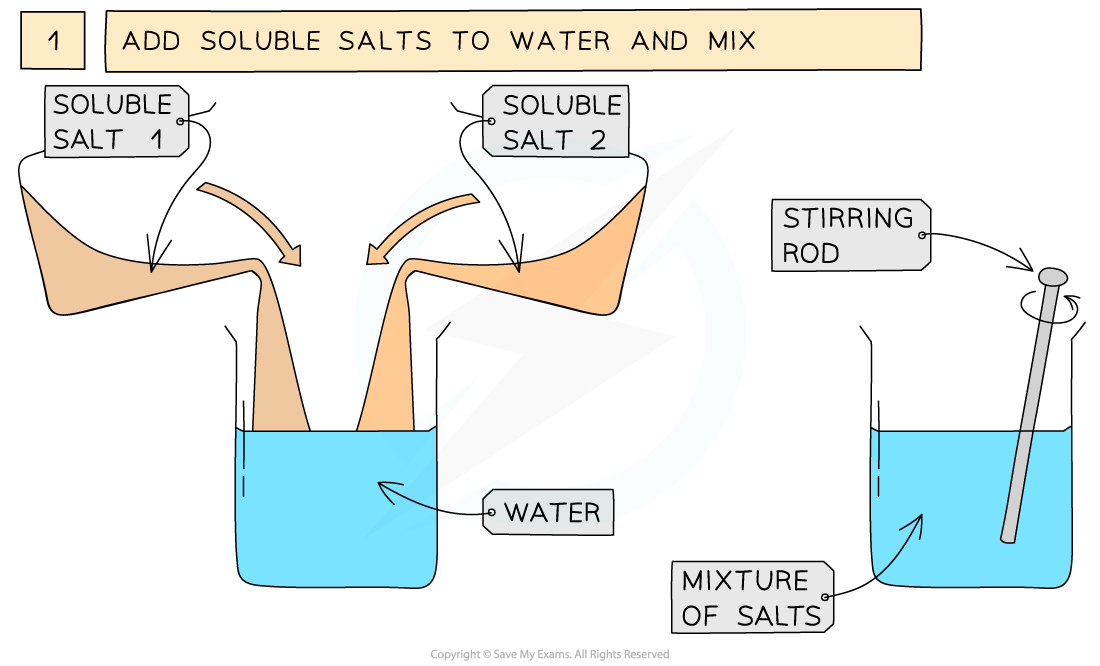
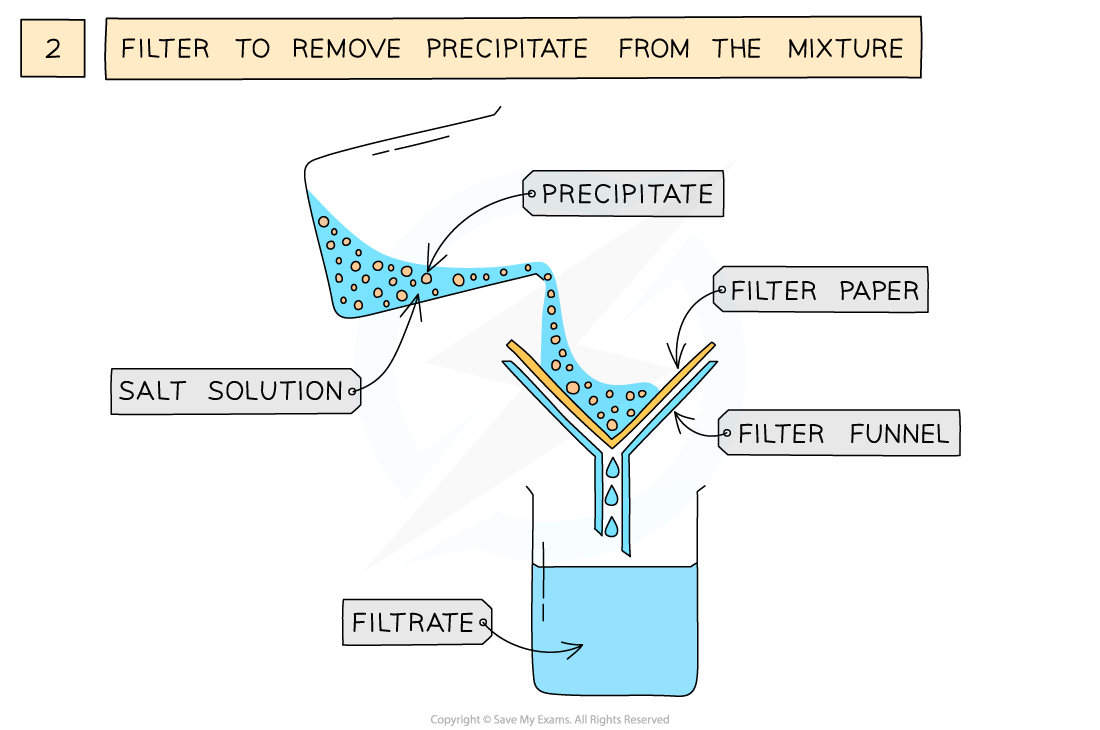
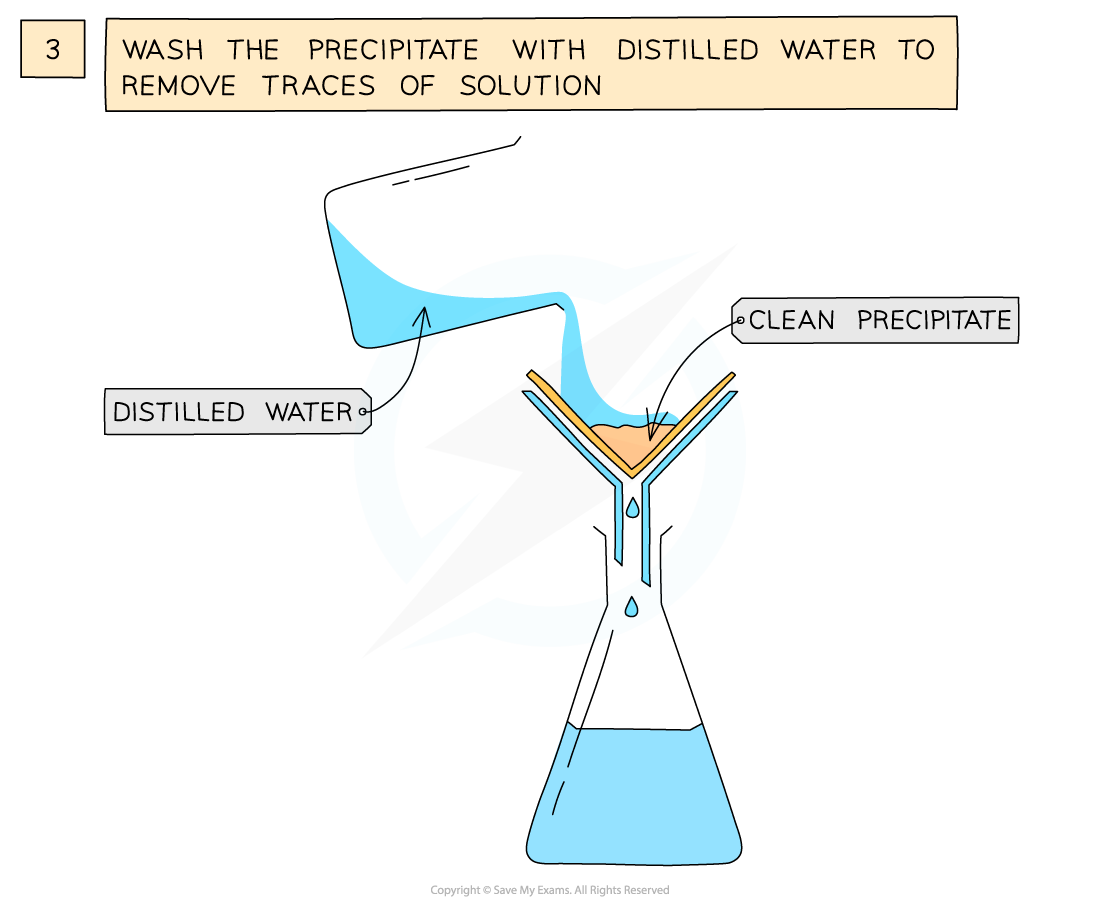
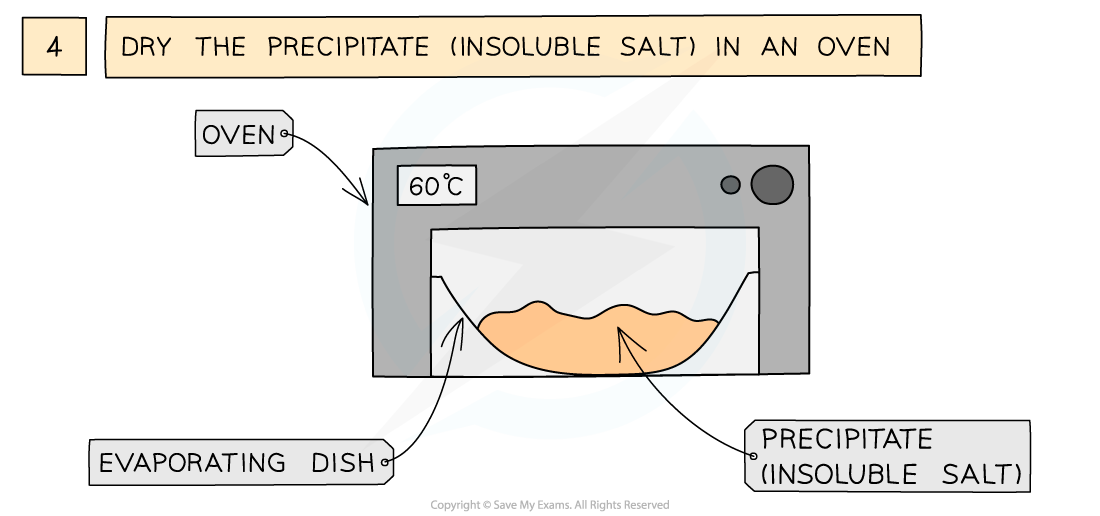
The preparation of lead(II)sulfate by precipitation from two soluble salts
Method:
- Measure out 25 cm3 of 0.5 mol dm3 lead(II)nitrate solution and add it to a small beaker
- Measure out 25 cm3 of 0.5 mol dm3 of potassium sulfate add it to the beaker and mix together using a stirring rod
- Filter to remove precipitate from mixture
- Wash filtrate with distilled water to remove traces of other solutions
- Leave in an oven to dry
Soluble salt 1 = lead(II) nitrate Soluble salt 2 = potassium sulfateEquation for the reaction:
Pb(NO3)2 (aq) + K2SO4 (aq) → PbSO4 (s) + 2KNO3 (aq)
lead(II) nitrate + potassium sulfate → lead(II) sulfate + potassium nitrate
Exam Tip
Care should be taken with handling lead salts as they are toxic.
转载自savemyexam

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








