- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 2.7.4 Bases
Edexcel IGCSE Chemistry 复习笔记 2.7.4 Bases
Bases
What makes a base act like a base?
Bases are substances which can neutralise an acid, forming a salt and water
The term base and alkali are not the same
So, all alkalis are bases, but not all bases are alkalisA base which is water-soluble is referred to as an alkali
Alkalis have pH values of above 7
In basic (alkaline) conditions red litmus paper turns blue
Bases are usually oxides, hydroxides or carbonates of metals
The presence of the OH- ions is what makes the aqueous solution an alkali
One unusual base is ammonia solution
When ammonia reacts with water it produces hydroxide ions
Some Common Alkalis and the Ions They Contain 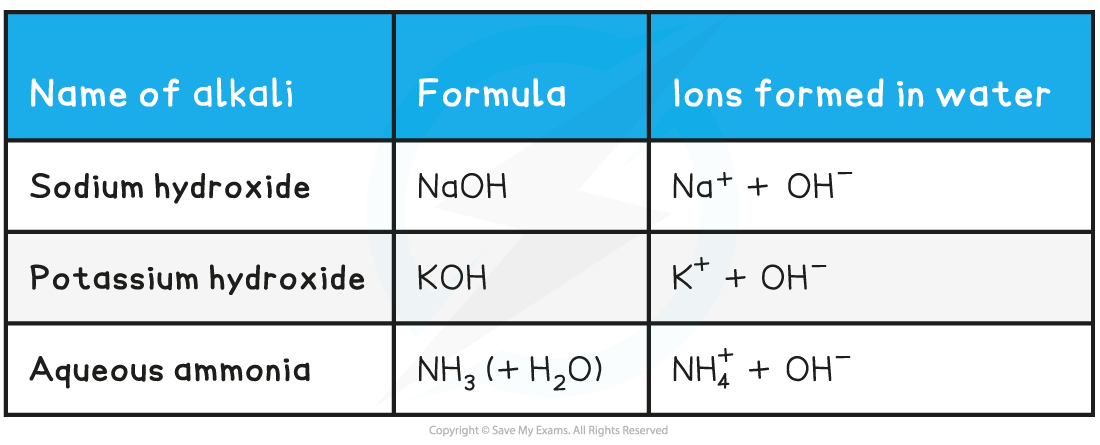 Exam Tip
Exam Tip
 Exam Tip
Exam TipAqueous ammonia and ammonium hydroxide are the same thing. When ammonia gas dissolves in water it forms ammonium hydroxide. Be careful to use the correct terminology: ammonia is the gas, NH3, ammonium is the ion present in ammonium compounds, NH4+
转载自savemyexam

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









