- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 2.7.3 Reactions of Acids
Edexcel IGCSE Chemistry 复习笔记 2.7.3 Reactions of Acids
Reactions of Acids
Reactions of acids with metals
- Only metals above hydrogen in the reactivity series will react with dilute acids
- The more reactive the metal then the more vigorous the reaction will be
- Metals that are placed high on the reactivity series such as potassium and sodium are very dangerous and react explosively with acids
- When acids react with metals they form a salt and hydrogen gas:
- The general equation is:
metal + acid ⟶ salt + hydrogen
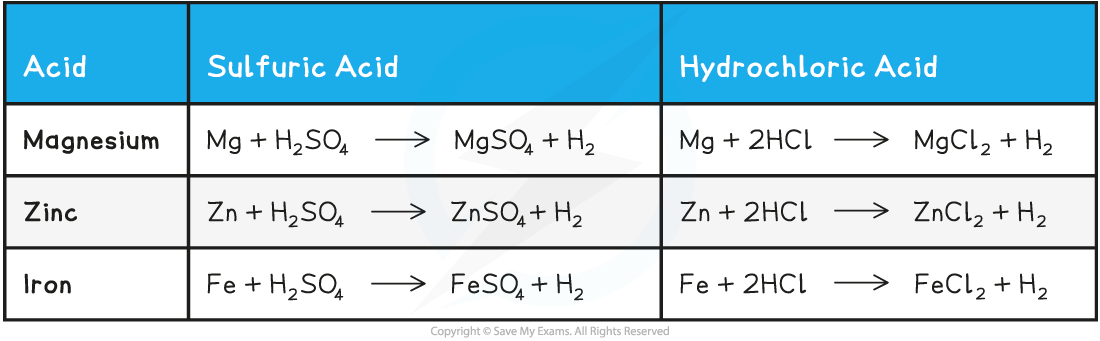
- Some examples of metal-acid reactions and their equations are given below:
Acid-Metals Reactions Table
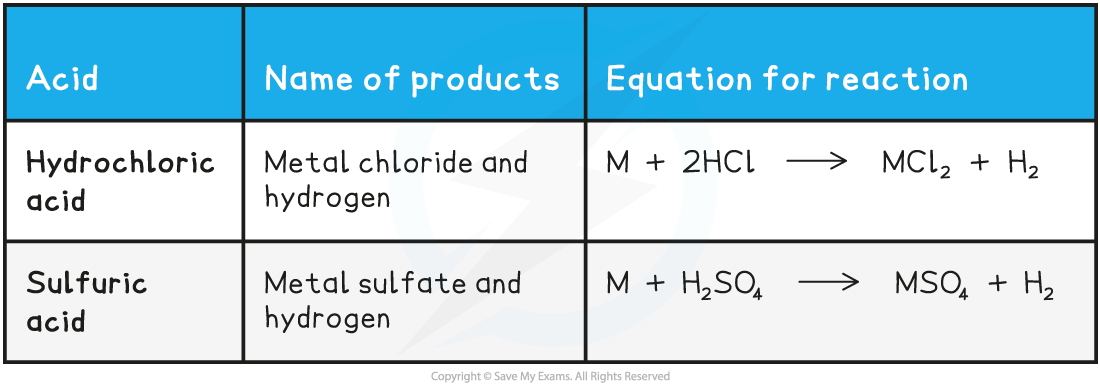
- In general, we can summarise the reaction of a metal that forms a +2 ion as follows:
Acids-Metals Summary Table
Reaction of acids with bases
- When an acid reacts with a base, a neutralisation reaction occurs
- In all acid-base neutralisation reactions, a salt and water are produced:
acid + base ⟶ salt + water
- The identity of the salt produced depends on the acid used and the positive ions in the base
- Hydrochloric acid produces chlorides, sulfuric acid produces sulfate salts and nitric acid produces nitrates
- Metal oxides and metal hydroxides act as bases
- The following are some specific examples of reactions between acids and metal oxides / hydroxides:
2HCl + CuO ⟶ CuCl2 + H2O
H2SO4 + 2NaOH ⟶ Na2SO4 + 2H2O
HNO3 + KOH ⟶ KNO3 + H2O
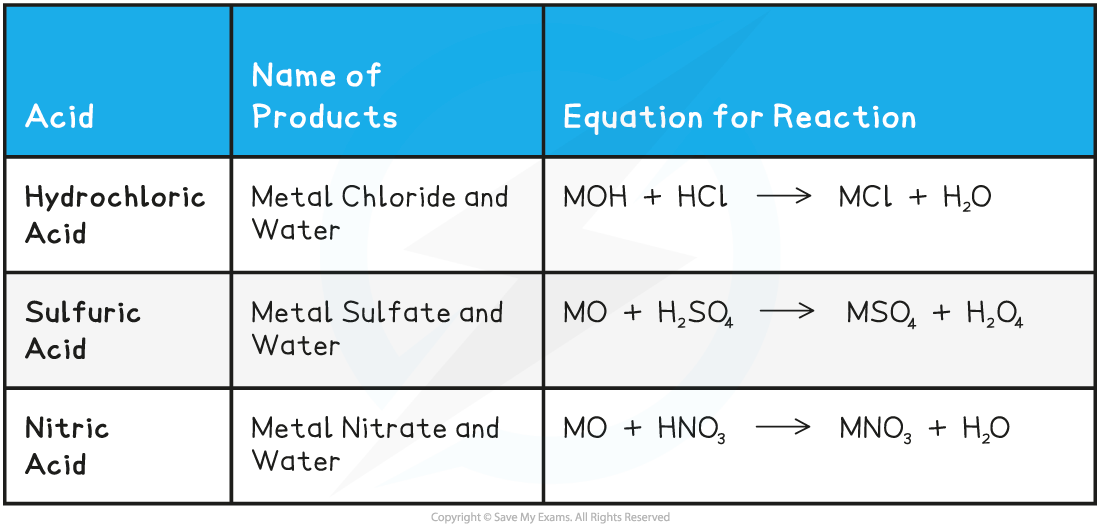
- In general, we can summarise the reaction of metals and bases as follows:
Acids and Metals Oxides or Hydroxides Summary Table
Reactions of Acids with Metal Carbonates
- Acids will react with metal carbonates to form the corresponding metal salt, carbon dioxide and water
- These reactions are easily distinguishable from acid – metal oxide/hydroxide reactions due to the presence of effervescence caused by the carbon dioxide gas
Acids & Metal Carbonates Reactions Table
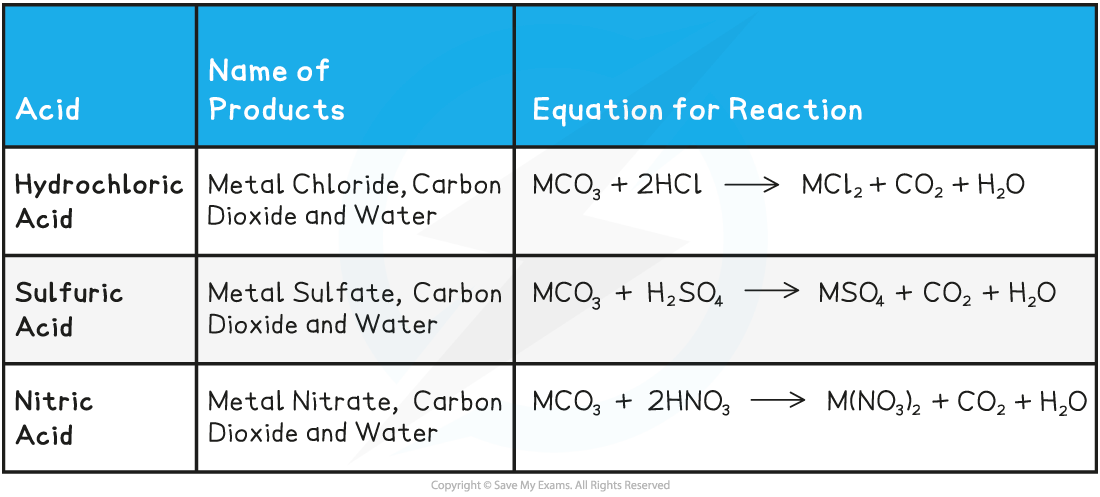
- The following are some specific examples of reactions between acids and metal carbonates:
2HCl + Na2CO3 ⟶ 2NaCl + H2O + CO2
H2SO4 + CaCO3⟶ CaSO4 + H2O + CO2
Exam Tip
If in an acid-base reaction there is effervescence produced then the base must be a metal carbonate which produces carbon dioxide gas.
转载自savemyexam


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








