- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 2.6.2 Acids, Alkalis & Neutralisation
Edexcel IGCSE Chemistry 复习笔记 2.6.2 Acids, Alkalis & Neutralisation
Acids & Alkalis
- When acids are added to water, they form positively charged hydrogen ions (H+)
- The presence of H+ ions is what makes a solution acidic
- When alkalis are added to water, they form negative hydroxide ions (OH–)
- The presence of the OH– ions is what makes the aqueous solution an alkali
- The pH scale is a numerical scale which is used to show how acidic or alkaline a solution is, in other words it is a measure of the amount of the ions present in solution
Neutralisation
- A neutralisation reaction occurs when an acid reacts with an alkali
- When these substances react together in a neutralisation reaction, the H+ ions react with the OH– ions to produce water
- For example, when hydrochloric acid is neutralised a sodium chloride and water are produced:
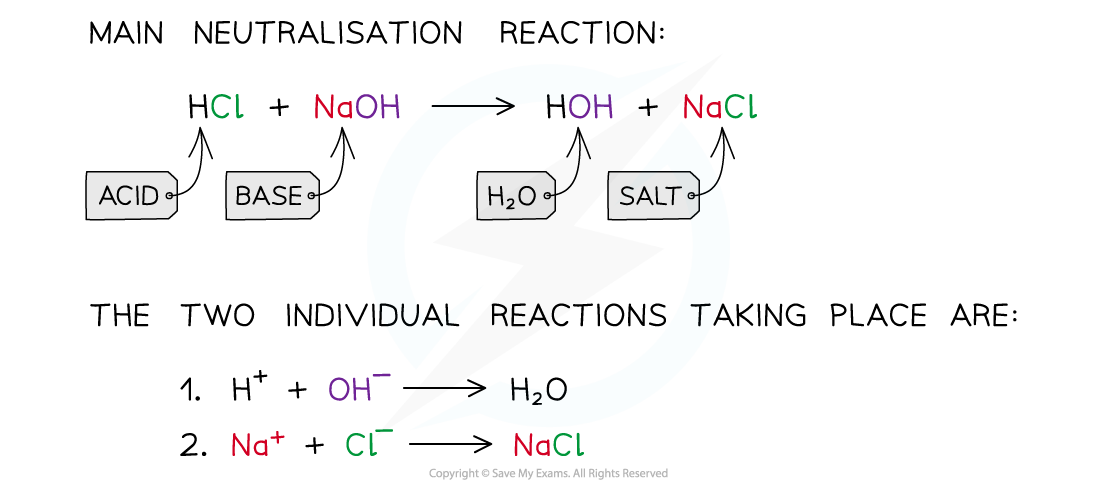
- The net ionic equation of all acid-base neutralisations and is what leads to a neutral solution, since water has a pH of 7:
H+ + OH– ⟶ H2O
- Neutralisation is very important in the treatment of soils to raise the pH as some crops cannot tolerate pH levels below 7
- This is achieved by adding bases to the soil such as limestone and quicklime
Exam Tip
Not all reactions of acids are neutralisations. For example, when a metal reacts with an acid, although a salt is produced there is no water formed so it does not fit the definition of neutralisation.
转载自savemyexam

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








