- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)
How Alkali Metals React with Water
- The group 1 metals are known as the alkali metals
- They form alkaline solutions when they react with water
- The group 1 metals are lithium, sodium, potassium, rubidium, caesium and francium and they are found in the first column of the periodic table
- The alkali metals share similar characteristic chemical properties because they each have one electron in their outermost shell
- Some of these properties are:
- They are all soft metals which can easily be cut with a knife
- They have relatively low densities and low melting points
- They are very reactive (they only need to lose one electron to become highly stable)

The alkali metals lie on the far left of the periodic table, in the very first group
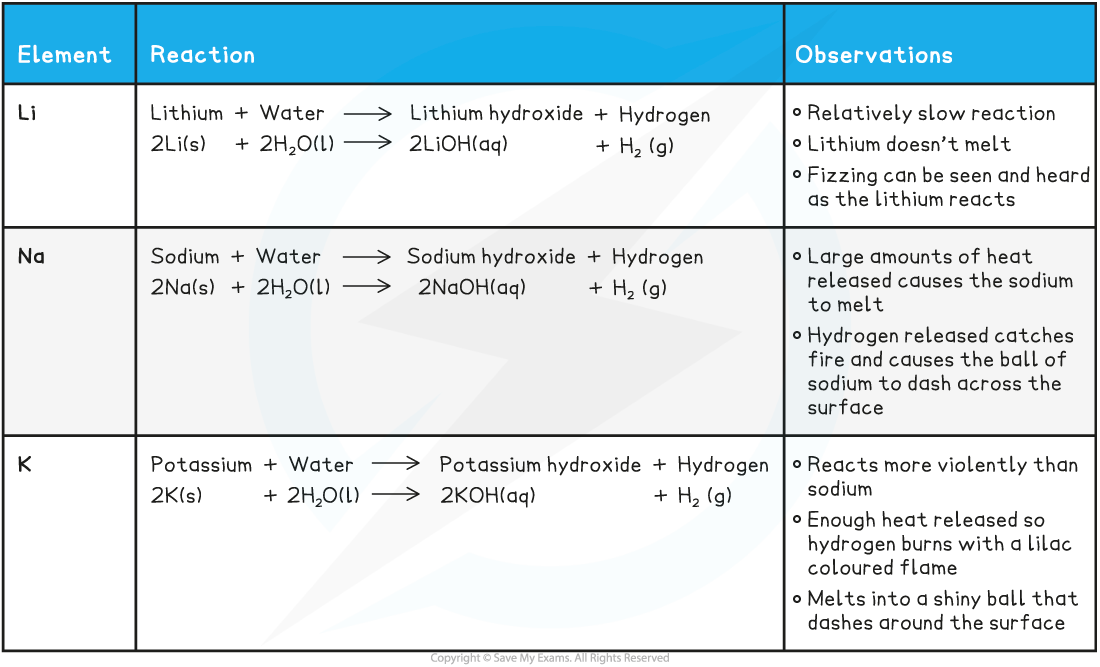
Reaction with water
- The reaction of the group 1 metals with water provides evidence for categorising these elements into the same chemical family
- The general pattern shown is:
group 1 metal + water ⟶ metal hydroxide + hydrogen
2M (s) + 2H2O (l) ⟶ 2MOH (aq) + H2 (g)
where M is Li, Na, K, Rb or Cs
- The hydroxides formed all have the same general formula and are colourless, aqueous solutions
- The metals are so named because they form alkalis in water
Exam Tip
Remember the group 1 metals all produce alkaline solutions (>pH 7) when they react with water.Lithium will produce a solution of lithium hydroxide; sodium will produce a solution of sodium hydroxide and so on.Make sure you can give the reaction equations with the correct state symbols to show what is happening during the reactions!
Trends in Group 1
- The differences between the reactions of the group 1 metals with water and oxygen provide evidence of trends within the group
Reactions with Water
- The reactions of the alkali metals with water get more vigorous as you descend the group
Summary of the Reactions of the First Three Alkali Metals with Water
Reactions with Oxygen
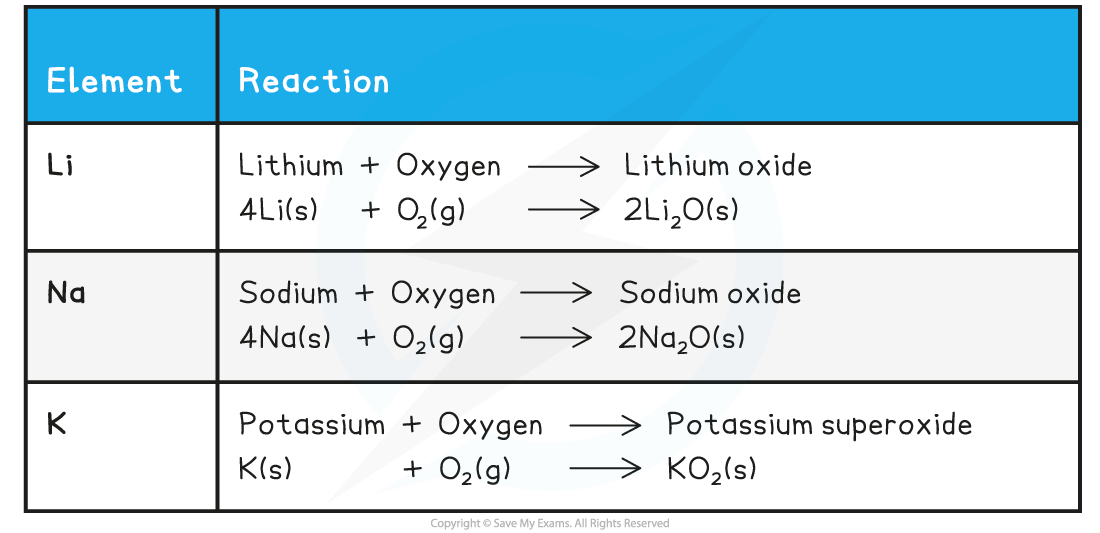
- The alkali metals react with oxygen in the air forming metal oxides, which is why the alkali metals tarnish when exposed to the air
- The metal oxide produced is a dull coating which covers the surface of the metal
- The metal tarnish more rapidly as you go down the group
Summary of the Reactions of the First Three Alkali Metals with Oxygen
Physical Trends
- Apart from the chemical trends there are also patterns to be seen in the physical properties
- The alkali metals are soft and easy to cut, getting softer as you move down the group
- The first three alkali metals are less dense than water
- They all have relatively low melting points which decrease as you move down the group, due to decreasing attractive forces between outer electrons and positive ions

The melting point of the group 1 metals decreases as you descend the group
Exam Tip
Trends are patterns of behaviour that change as you go down a group or across a period. Trends are not the same as rules, so sometimes there are odd properties that seem inconsistent, but the overall patterns remain the same.
Predicting Properties in Group 1
- Following these trends, we can say that:
- Rubidium, caesium and francium will react even more vigorously with air and water than the first three alkali metals
- Of the alkali metals, lithium is the least reactive (as it is at the top of group 1) and francium would be the most reactive (as it’s at the bottom of group 1)
- Using the information given in the trends we would predict that rubidium:
- would be a soft grey solid
- appears shiny when freshly cut
- is more dense than potassium (> 0.86 g cm-3)
- has a lower melting point than potassium (< 63.5 oC)
Exam Tip
You could be asked to make predictions about how rubidium would be expected to react with water, knowing that it lies below potassium in group 1. Words like 'explosively' and 'violently' would be good ones to choose when describing the reaction.
转载自savemyexam

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








