Practical: Investigate the Electrolysis of Aqueous Solutions
Aim:
To electrolyse aqueous solutions of sodium chloride, sulfuric acid and copper(II)sulfate, and to collect and identify the products at each electrode
Diagram: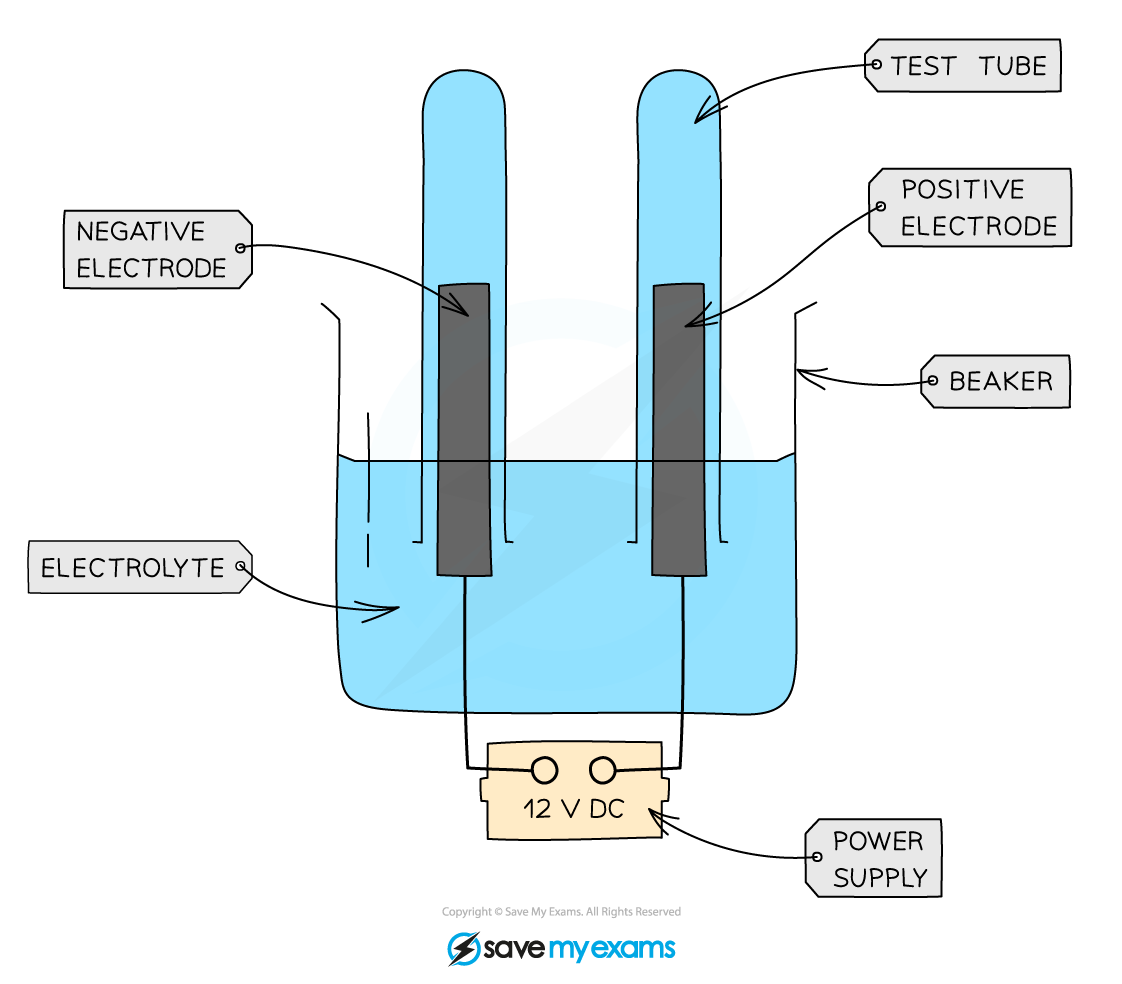
Electrolysis cell for collecting gaseous products from aqueous solutions
Method:
- Add the aqueous solution to a beaker and cover the electrodes with the solution
- Invert two small test tubes to collect any gaseous products
- Connect the electrodes to a power pack or battery.
- Turn on the power pack or battery and allow electrolysis to take place
- Observations at each electrode are made
- Gases collected in the test tube can be tested and identified
- If the gas produced at the cathode burns with a ‘pop’ when a sample is lit with a lighted splint. This shows that the gas is hydrogen
- If the gas produced at the anode relights a glowing splint dipped into a sample of the gas. This shows that the gas is oxygen
- If the anode gas bleaches of a piece of litmus paper this indicates chlorine is the product
- If a solid forms around the electrode, the metal have been formed. The colour can indicate the metal
Results:
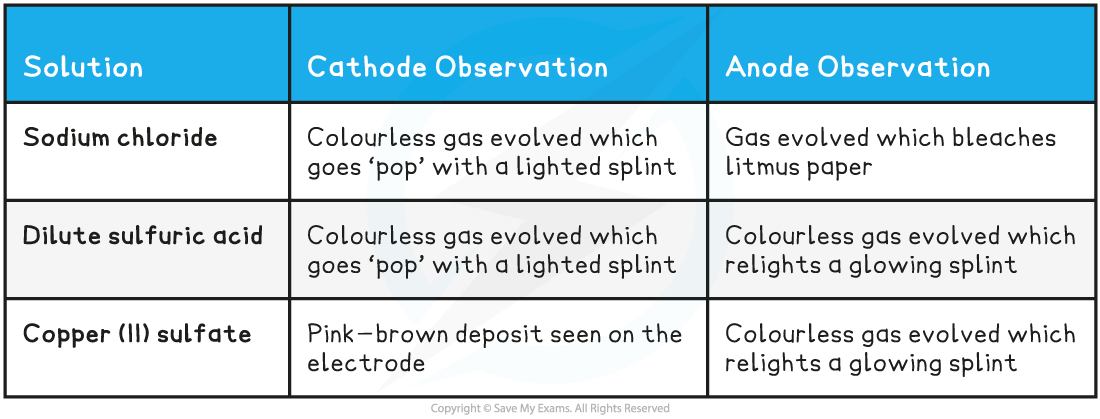
Conclusions:
- Sodium chloride solutions produces hydrogen at the cathode and chlorine at the anode
- Dilute sulfuric acid produces hydrogen at the cathode and oxygen at the anode
- Copper(II)sulfate solution produces copper at the cathode an oxygen at the anode
转载自savemyexam










