- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记8.3.4 Nuclear Radius
Estimating Nuclear Radius
Closest Approach Method
- In the Rutherford scattering experiment, alpha particles are fired at a thin gold foil
- Some of the alpha particles are found to come straight back from the gold foil
- This indicates that there is electrostatic repulsion between the alpha particles and the gold nucleus
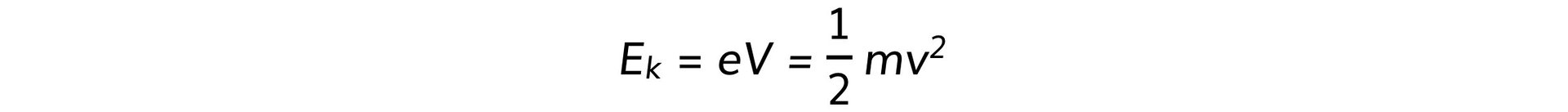
- At the point of closest approach, r, the repulsive force reduces the speed of the alpha particles to zero momentarily
- At this point, the initial kinetic energy of an alpha particle, Ek, is equal to electric potential energy, Ep
- The radius of the closest approach can be found be equating the initial kinetic energy to the electric potential energy
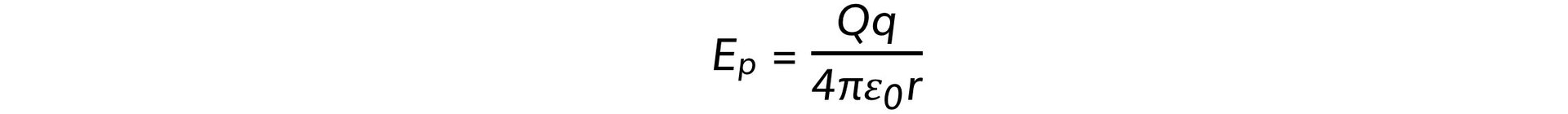
- Equating the two equations gives:
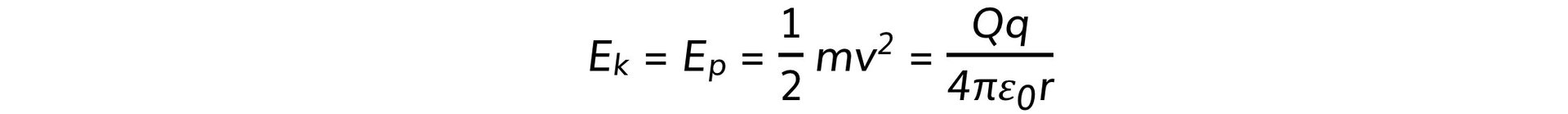

Pros & Cons of Closest Approach Method
Advantages
- Alpha scattering gives a good estimate of the upper limit for a nuclear radius
- The mathematics behind this approach are very simple
- The alpha particles are scattered only by the protons and not all the nucleons that make up the nucleus
Disadvantages
- This method does not give an accurate value for nuclear radius as it will always be an overestimate
- This is because it measures the nearest distance the alpha particle can get to the gold nucleus, not the radius of it
- Alpha particles are hadrons, therefore, when they get close to the nucleus they are affected by the strong nuclear force and the mathematics do not account for this
- The gold nucleus will recoil as the alpha particle approaches
- Alpha particles have a finite size whereas electrons can be treated as a point mass
- It is difficult to obtain alpha particles which rebound at exactly 180°
- In order to do this, a small collision region is required
- The alpha particles in the beam must all have the exact same initial kinetic energy
- The sample must be extremely thin to prevent multiple scattering
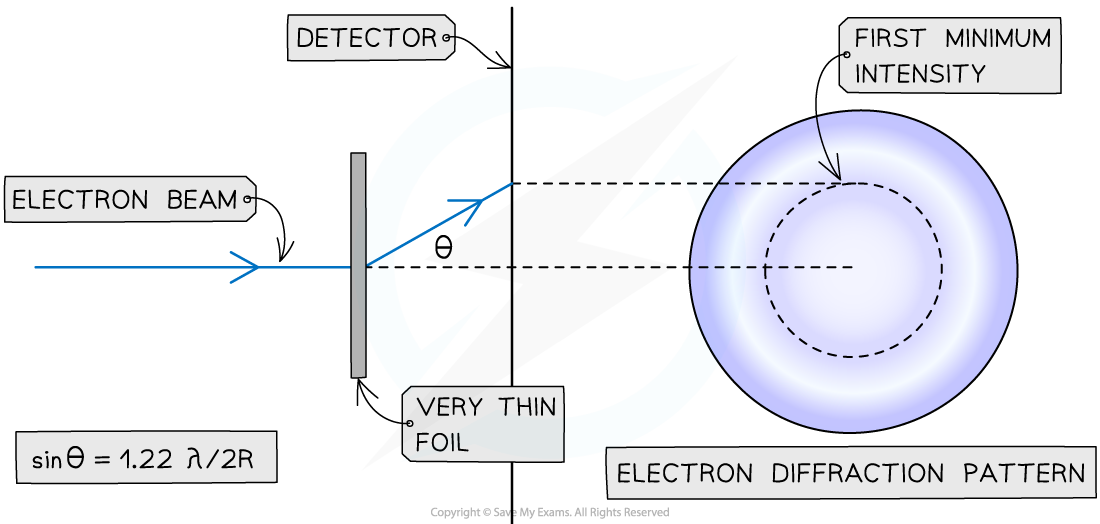
Electron Diffraction Method
- Electrons accelerated to close to the speed of light have wave-like properties such as the ability to diffract and have a de Broglie wavelength equal to:
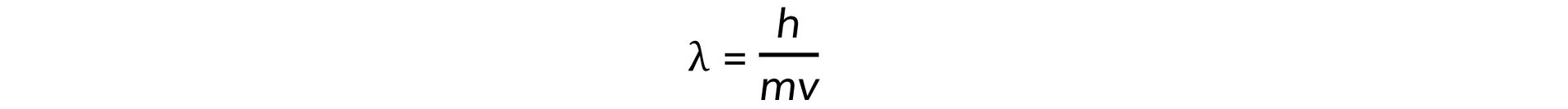
- Where:
- h = Planck's constant
- m = mass of an electron (kg)
- v = speed of the electrons (m s−1)
- The diffraction pattern forms a central bright spot with dimmer concentric circles around it
- From this pattern, a graph of intensity against diffraction angle can be used to find the diffraction angle of the first minimum
- Using this, the size of the atomic nucleus, R, can be determined from:
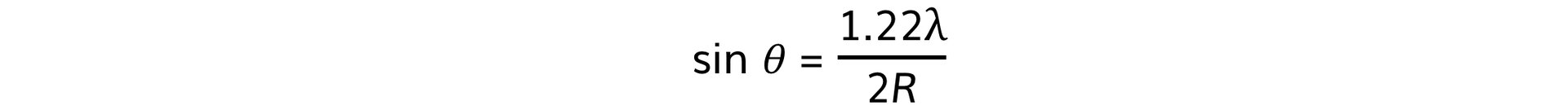
- Where:
- θ = angle of the first minimum (degrees)
- λ = de Broglie wavelength (m)
- R = radius of the nucleus (m)
Pros & Cons of Electron Diffraction Method
Advantages
- Electron diffraction is much more accurate than the closest approach method
- This method gives a direct measurement of the radius of a nucleus
- Electrons are leptons; therefore, they will not interact with nucleons in the nucleus through the strong nuclear force as an alpha particle would
Disadvantages
- Electrons must be accelerated to very high speeds to minimise the de Broglie wavelength and increase resolution
- This is because significant diffraction takes place when the electron wavelength is similar in size to the nuclear diameter
- Electrons can be scattered by both protons and neutrons
- If there is an excessive amount of scattering, then the first minimum of the electron diffraction can be difficult to determine
Electron Diffraction by a Nucleus
- The graph of intensity against angle obtained through electron diffraction is as follows:

Worked Example
The graph shows how the relative intensity of the scattered electrons varies with angle due to diffraction by the oxygen-16 nuclei. The angle is measured from the original direction of the beam.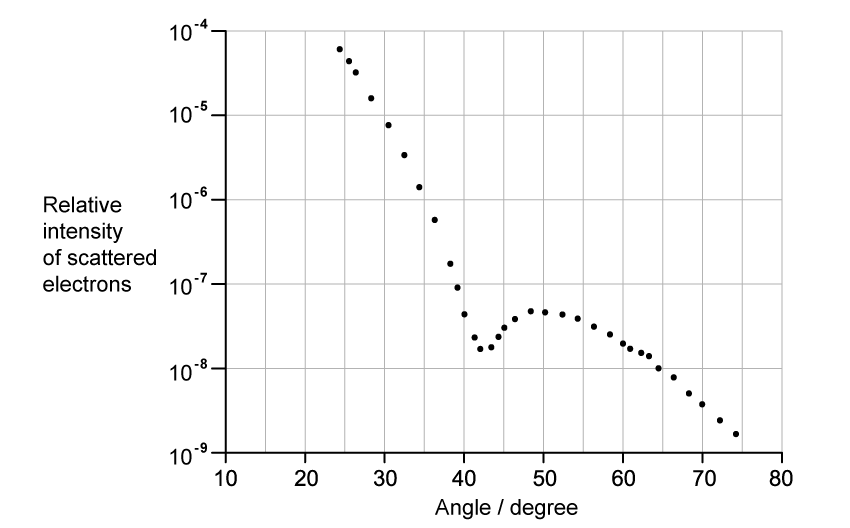 The de Broglie wavelength λ of each electron in the beam is 3.35 × 10−15 m.Calculate the radius of an oxygen-16 nucleus using information from the graph.
The de Broglie wavelength λ of each electron in the beam is 3.35 × 10−15 m.Calculate the radius of an oxygen-16 nucleus using information from the graph.
Step 1: Identify the first minimum from the graph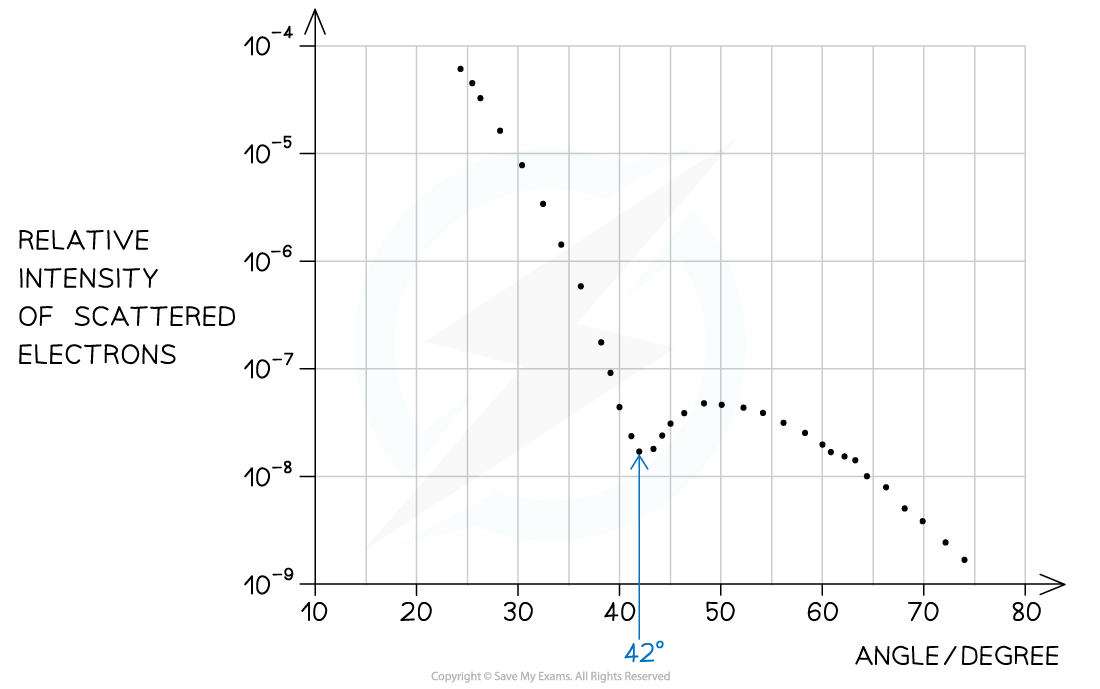
- Angle of first minimum, θ = 42°
Step 2: Write out the equation relating the angle, wavelength, and nuclear radius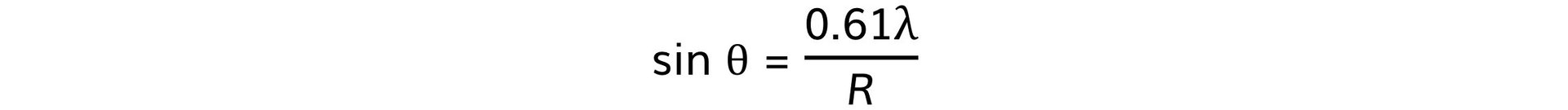 Step 3: Calculate the nuclear radius, R
Step 3: Calculate the nuclear radius, R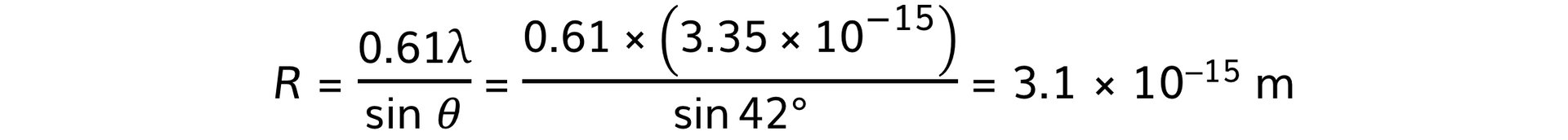
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









