- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记8.3.2 Decay Equations
Changes in N and Z by Radioactive Decay
- There are four reasons why a nucleus might become unstable, and these determine which decay mode will occur
- Too many neutrons
- Decays through beta-minus (β-) emission
- One of the neutrons in the nucleus changes into a proton and a β- particle (an electron) and antineutrino is released
- The nucleon number is constant
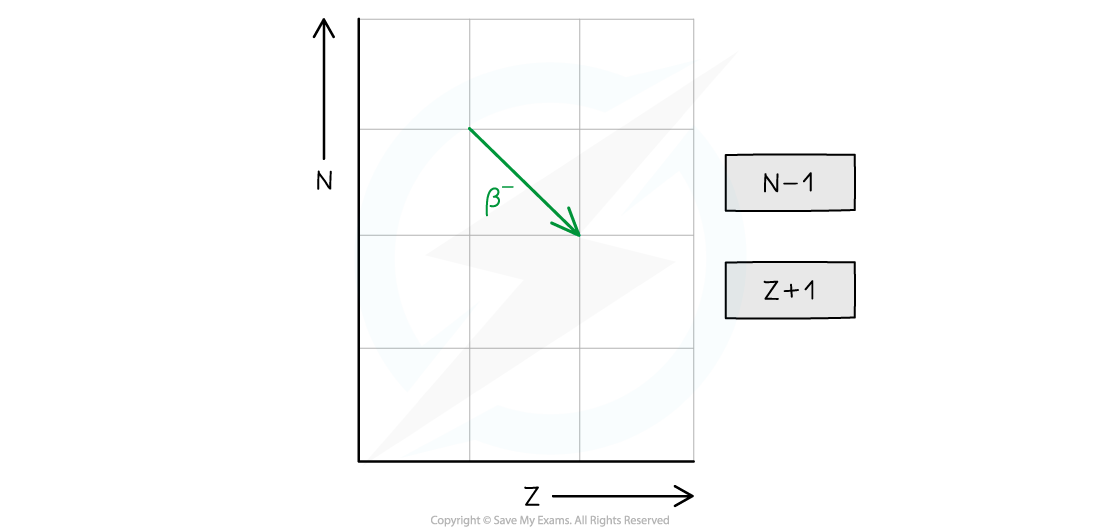
- The neutron number (N) decreases by 1
- The proton number (Z) increases by 1
- The general decay equation for β- emission is:
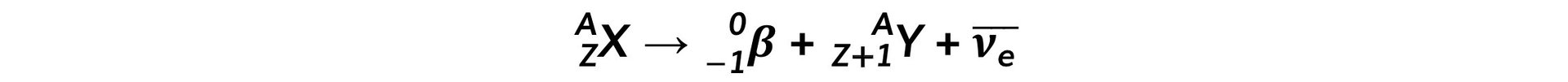
- Too many protons
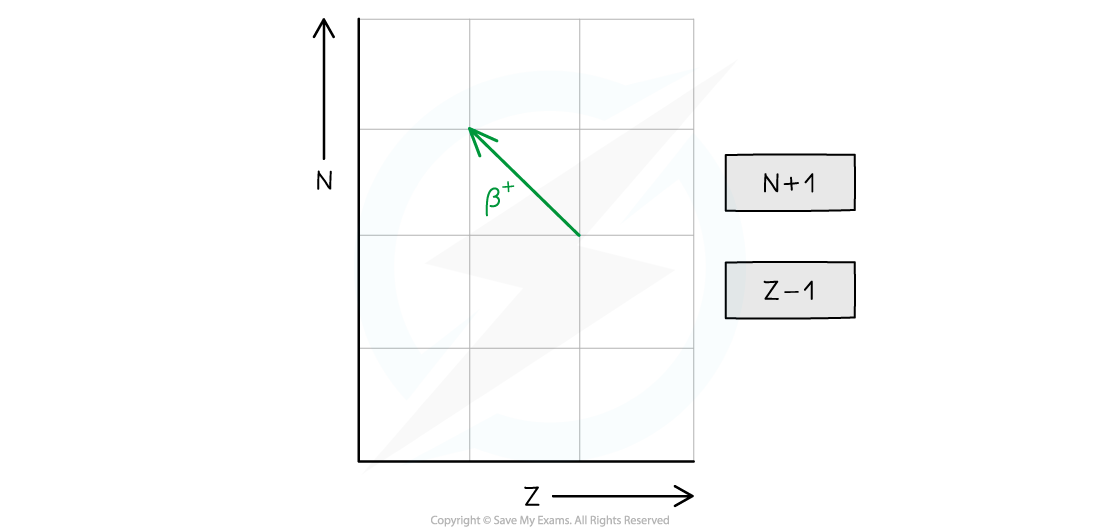
- Decays through beta-plus (β+) emission or electron capture
- In beta-plus decay, a proton changes into a neutron and a β+ particle (a positron) and neutrino are released
- In electron capture, an orbiting electron is taken in by the nucleus and combined with a proton causing the formation of a neutron and neutrino
- In both types of decay, the nucleon number stays constant
- The neutron number (N) increases by 1
- The proton number (Z) decreases by 1
- The general decay equation for β+ emission is:
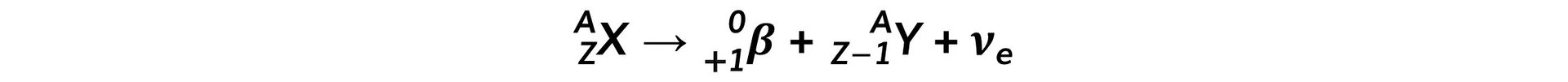
-
- The equation for electron capture is:
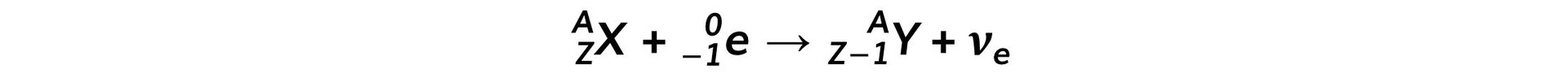
- Too many nucleons
- Decays through alpha (α) emission
- An α particle is a helium nucleus
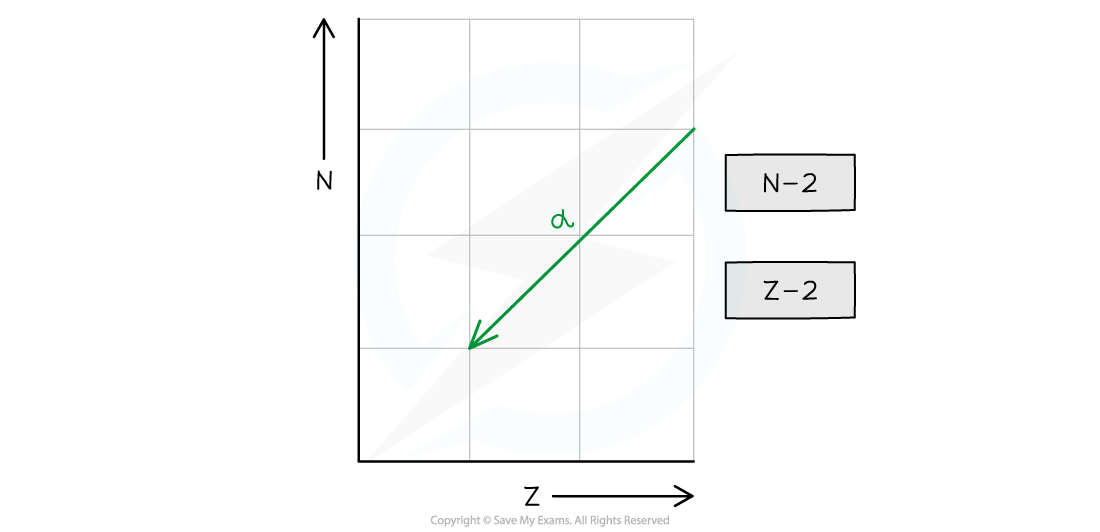
- The nucleon number decreases by 4 and the proton number decreases by 2
- The neutron number (N) decreases by 2
- The proton number (Z) decreases by 2
- The general decay equation for α emission is:
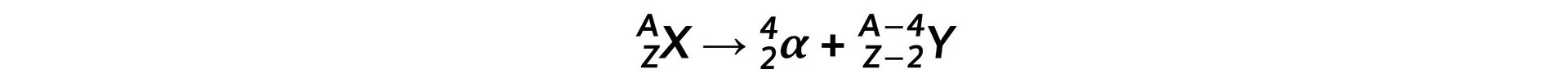
- Too much energy
- Decays through gamma (γ) emission
- A gamma particle is a high-energy electromagnetic radiation
- This usually occurs after a different type of decay, such as alpha or beta decay
- This is because the nucleus becomes excited and has excess energy
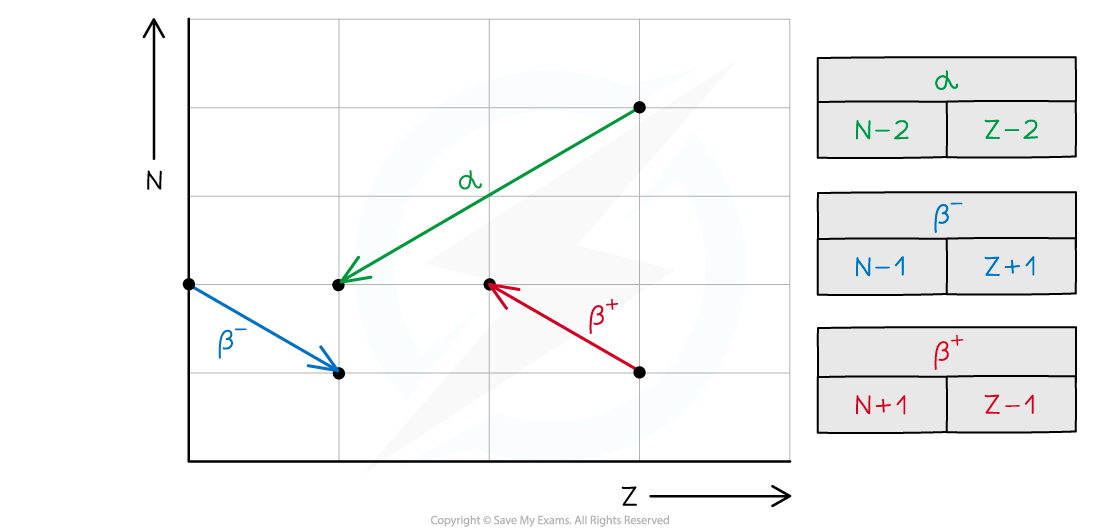
- In summary, alpha decay, beta decay and electron capture can be represented on an N–Z graph as follows:
Worked Example
Plutonium-239 is a radioactive isotope that contains 94 protons and emits α particles to form a radioactive isotope of uranium. This isotope of uranium emits α particles to form an isotope of thorium which is also radioactive.
a) Write two equations to represent the decay of plutonium-239 and the subsequent decay of uranium
b) Predict the decay mode of the thorium isotope
c) Draw the decay chain from plutonium-239 to the daughter product of thorium decay on an N–Z graph
Part (a)Step 1: Write down the general equation of alpha decay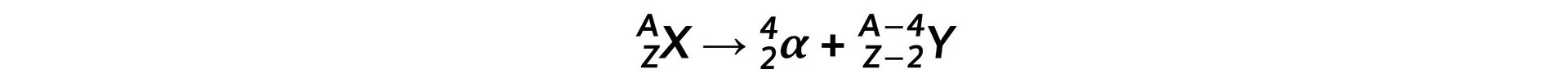 Step 2: Write down the decay equation of plutonium into uranium
Step 2: Write down the decay equation of plutonium into uranium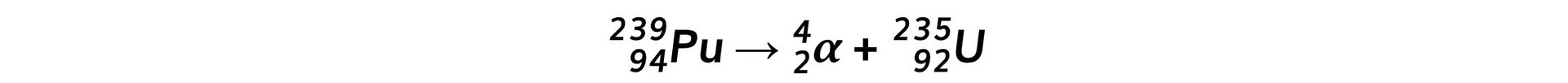 Step 3: Write down the decay equation of uranium into thorium
Step 3: Write down the decay equation of uranium into thorium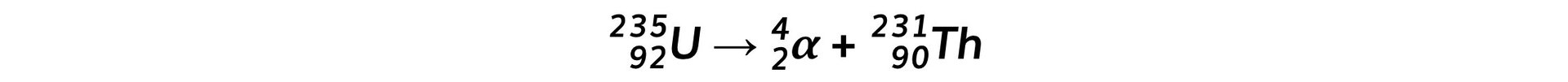
Part (b)
- Plutonium, 239Pu
- Number of neutrons: 239 – 94 = 145
- Neutron-nucleon ratio: 145 / 239 = 0.607
- Uranium, 235U
- Number of neutrons: 235 – 92 = 143
- Neutron-nucleon ratio: 143 / 235 = 0.609
- Thorium, 231Th
- Number of neutrons: 231 – 90 = 141
- Neutron-nucleon ratio: 141 / 231 = 0.610
- Thorium-231 is neutron-rich compared to uranium-235 and plutonium-239
- Therefore, it must be a β– emitter
Part (c)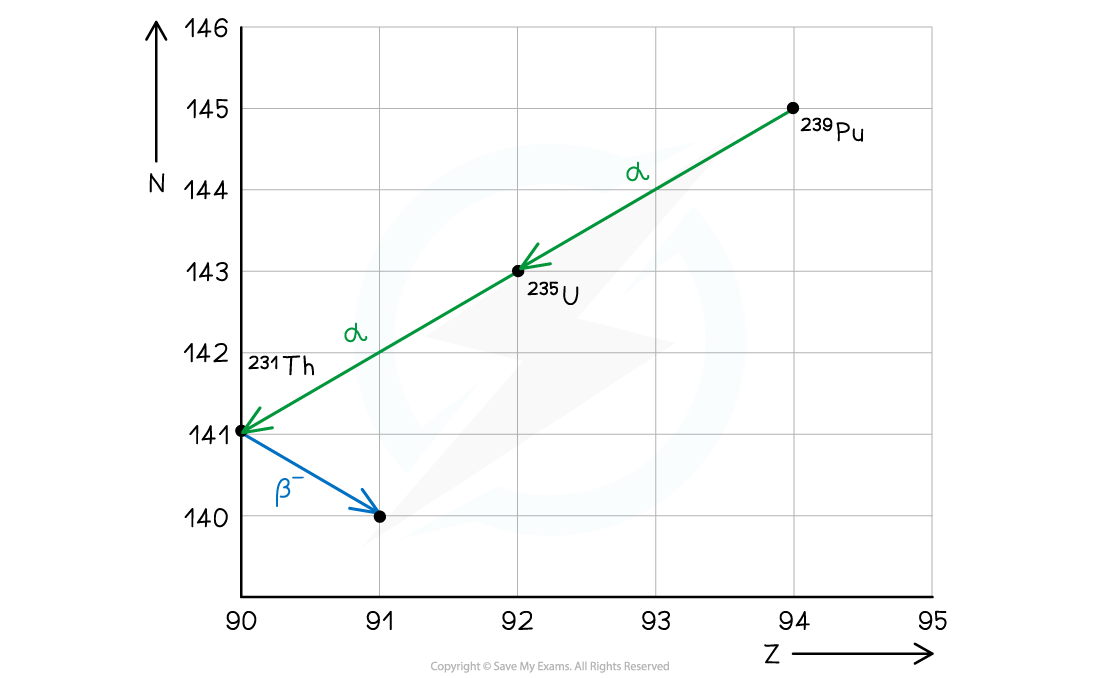
- The key features to draw on an N–Z graph are:
- Values for neutron number (N) on the vertical axis
- Values for proton number (Z) on the horizontal axis
- Labels for the isotopes eg. 239Pu, 235U, 231Th
- Arrows showing the direction of the decay
- Labels for the type of emission eg. α, β–
Exam Tip
Watch out for the vertical axis for the N-Z graph. Instead of N for number of neutrons this is sometimes labelled as N for nucleon number (total protons and neutrons) which means the decays will be represented slightly differently.
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








