- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记8.2.1 Radioactive Decay
Radioactive Decay
- Radioactive decay is defined as:
The spontaneous disintegration of a nucleus to form a more stable nucleus, resulting in the emission of an alpha, beta or gamma particle
- Radioactive decay is a random process, this means that:
- There is an equal probability of any nucleus decaying
- It cannot be known which particular nucleus will decay next
- It cannot be known at what time a particular nucleus will decay
- The rate of decay is unaffected by the surrounding conditions
- It is only possible to estimate the proportion of nuclei decaying in a given time period
- The random nature of radioactive decay can be demonstrated by observing the count rate of a Geiger-Muller (GM) tube
- When a GM tube is placed near a radioactive source, the counts are found to be irregular and cannot be predicted
- Each count represents a decay of an unstable nucleus
- These fluctuations in count rate on the GM tube provide evidence for the randomness of radioactive decay
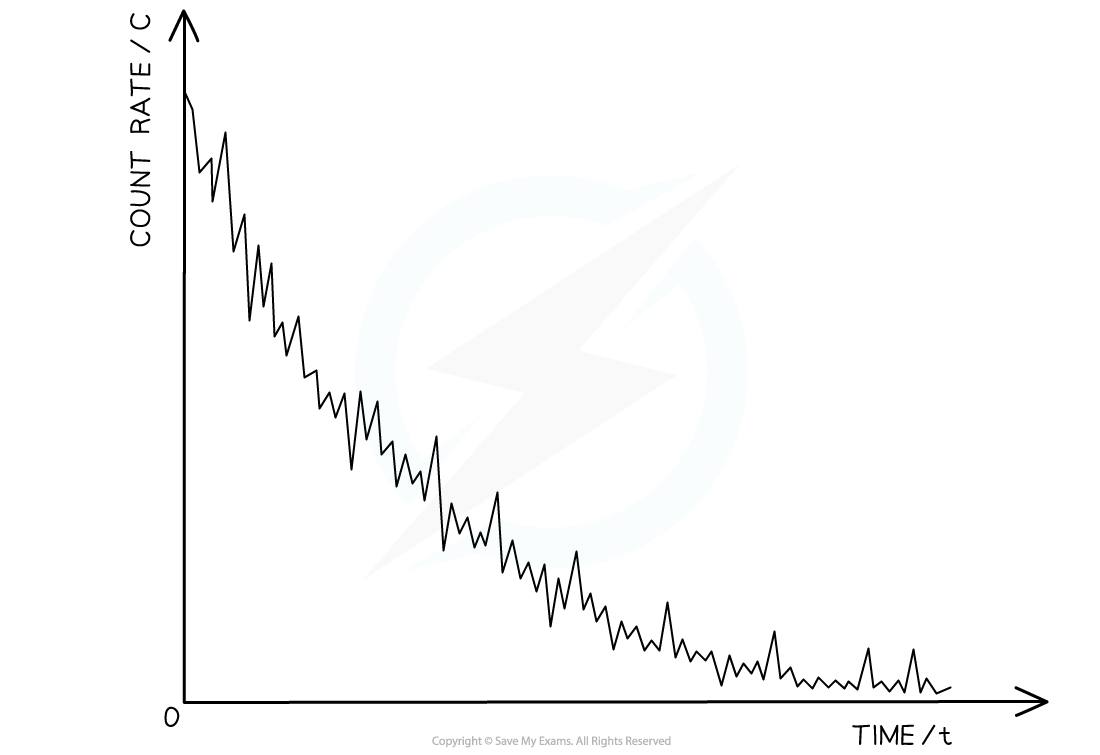
The variation of count rate over time of a sample radioactive gas. The fluctuations show the randomness of radioactive decay
Activity & The Decay Constant
- Since radioactive decay is spontaneous and random, it is useful to consider the average number of nuclei that are expected to decay per unit time
- This is known as the average decay rate
- As a result, each radioactive element can be assigned a decay constant
- The decay constant λ is defined as:
The probability that an individual nucleus will decay per unit of time
- When a sample is highly radioactive, this means the number of decays per unit time is very high
- This suggests it has a high level of activity
- Activity, or the number of decays per unit time can be calculated using:
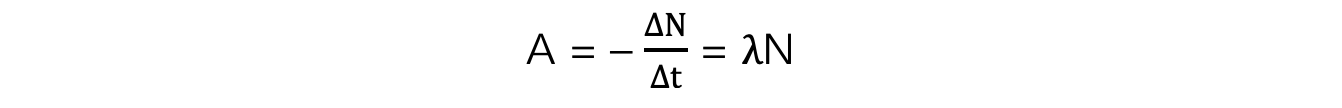
- Where:
- A = activity of the sample (Bq)
- ΔN = number of decayed nuclei
- Δt = time interval (s)
- λ = decay constant (s-1)
- N = number of nuclei remaining in a sample
- The activity of a sample is measured in Becquerels (Bq)
- An activity of 1 Bq is equal to one decay per second, or 1 s-1
- This equation shows:
- The greater the decay constant, the greater the activity of the sample
- The activity depends on the number of undecayed nuclei remaining in the sample
- The minus sign indicates that the number of nuclei remaining decreases with time
Worked Example
Radium is a radioactive element first discovered by Marie and Pierre Curie. They used the radiation emitted from radium-226 to define a unit called the Curie (Ci) which they defined as the activity of 1 gram of radium.It was found that in a 1 g sample of radium, 2.22 × 1012 atoms decayed in 1 minute.Another sample containing 3.2 × 1022 radium-226 atoms had an activity of 12 Ci.
a) Determine the value of 1 Curie
b) Determine the decay constant for radium-226
Part a)
Step 1: Write down the known quantities
-
- Number of atoms decayed, ΔN = 2.22 × 1012
- Time, Δt = 1 minutes = 60 s
Step 2: Write down the activity equation
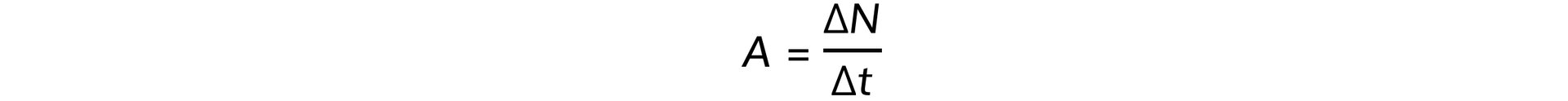 Step 3: Calculate the value of 1 Ci
Step 3: Calculate the value of 1 Ci
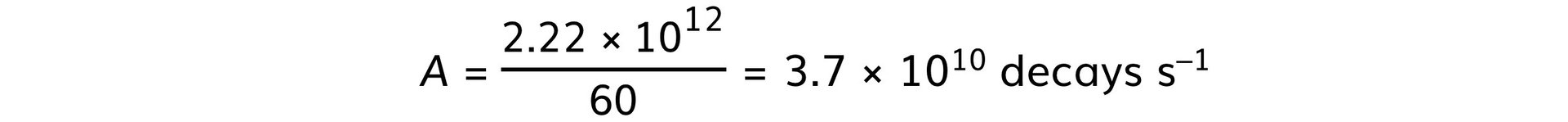
Part b)
Step 1: Write down the known quantities
-
- Number of atoms, N = 3.2 × 1022
- Activity, A = 12 Ci = 12 × (3.7 × 1010) = 4.44 × 1011 Bq
Step 2: Write down the activity equation
A = λN
Step 3: Calculate the decay constant of radium
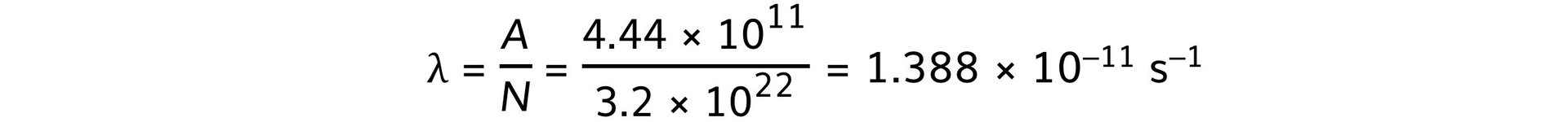
-
- Therefore, the decay constant of radium-226 is 1.4 × 10–11 s–1
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









