- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.7.4 Giant Covalent Structures
Edexcel IGCSE Chemistry 复习笔记 1.7.4 Giant Covalent Structures
Giant Covalent Structures
- Covalent bonding can be responsible for substances that have many different structures and therefore different physical properties
- We have already seen how small molecules such as H2O and N2 are simple units made from covalently bonded atoms
- These simple molecules contain fixed numbers of atoms
- Giant covalent structures on the other hand have a huge number of non-metal atoms bonded to other non-metal atoms via strong covalent bonds
- These structures can also be called giant lattices and have a fixed ratio of atoms in the overall structure
- Three common macromolecules you should know about are diamond, graphite and C60 fullerene
Exam Tip
Giant covalent structures can also be called macromolecules.
Diamond, Graphite & C60 Fullerene
Diamond
- Diamond and graphite are allotropes of carbon
- Both substances contain only carbon atoms but due to the differences in bonding arrangements they are physically completely different
- In diamond, each carbon atom bonds with four other carbons, forming a tetrahedron
- All the covalent bonds are identical, very strong and there are no intermolecular forces
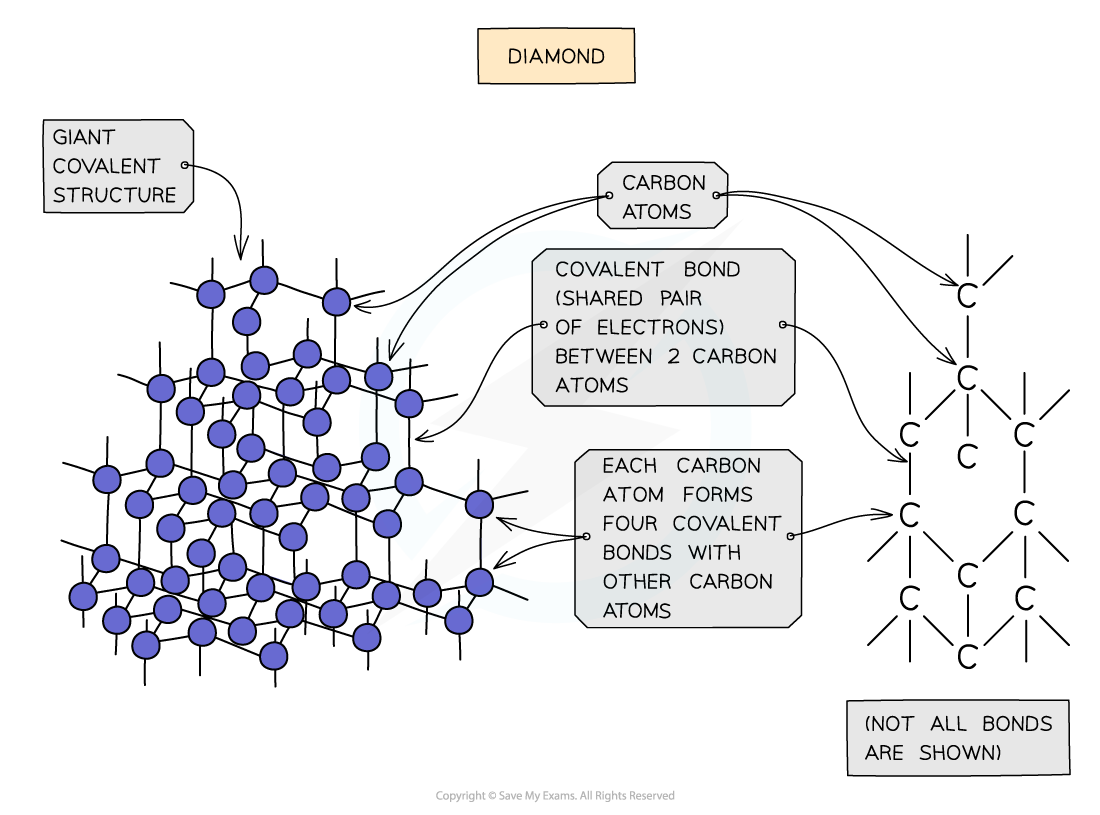
Diagram showing the structure and bonding arrangement in diamond
Properties of Diamond
- Diamond has the following physical properties:
- It does not conduct electricity
- It has a very high melting point
- It is extremely hard and has a density of 3.51 g / cm3 – a little higher than that of aluminium
- All the outer shell electrons in carbon are held in the four covalent bonds around each carbon atom, so there are no freely moving charged particles to the current
- The four covalent bonds are very strong and extend in a giant lattice, so a very large amount of heat energy is needed to break the lattice
- Diamond ́s hardness makes it very useful for purposes where extremely tough material is required
- Diamond is used in jewellery and for coating blades in cutting tools
- The cutting edges of discs used to cut bricks and concrete are tipped with diamonds
- Heavy-duty drill bits and tooling equipment are also diamond tipped
Exam Tip
Diamond is the hardest naturally occurring mineral, but it is by no means the strongest. Students often confuse hard with strong, thinking it is the opposites of weak. Diamonds are hard, but brittle – that is, they can be smashed fairly easily with a hammer. The opposite of saying a material is hard is to describe it as soft.
Graphite
- Each carbon atom in graphite is bonded to three others forming layers of hexagons, leaving one free electron per carbon atom
- These free electrons migrate along the layers and are free to move and carry charge, hence graphite can conduct electricity
- The covalent bonds within the layers are very strong, but the layers are attracted to each other by weak intermolecular forces, so the layers can slide over each other making graphite soft and slippery
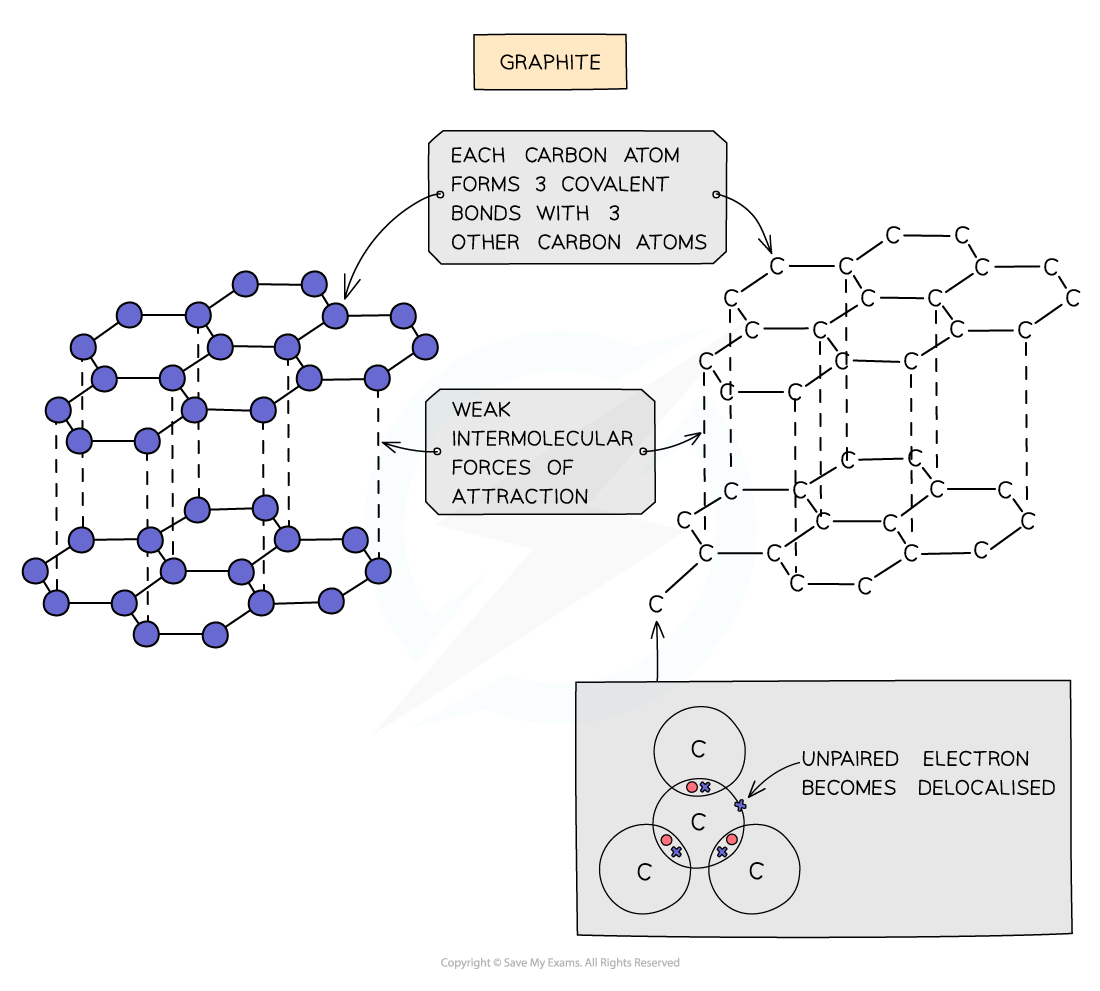
The structure and bonding in graphite
Properties of Graphite
- Graphite has the following physical properties:
- It conducts electricity and heat
- It has a very high melting point
- It is soft and slippery and less dense than diamond (2.25 g / cm3)
- The weak intermolecular forces make it a useful material
- It is used in pencils and as an industrial lubricant, in engines and in locks
- It is also used to make inert electrodes for electrolysis, which is particularly important in the extraction of metals such as aluminium
C60 fullerene
- Fullerenes are a group of carbon allotropes which consist of molecules that form hollow tubes or spheres
- Fullerenes can be used to trap other molecules by forming around the target molecule and capturing it, making them useful for targeted drug delivery systems
- They also have a huge surface area and are useful for trapping catalyst molecules onto their surfaces making them easily accessible to reactants so catalysis can take place
- Some fullerenes are excellent lubricants and are starting to be used in many industrial processes
- The first fullerene to be discovered was buckminsterfullerene which is affectionately referred to as a “buckyball”
- In this fullerene, 60 carbon atoms are joined together forming 20 hexagons and 12 pentagons which produce a hollow sphere that is the exact shape of a soccer ball
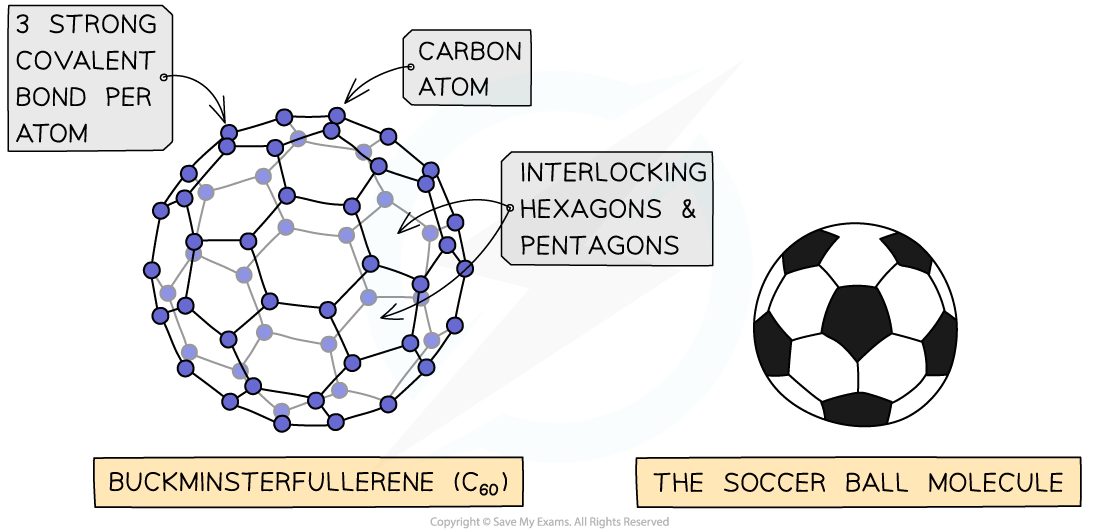
The structure n bonding in C60 fullerene - the football shaped molecule
Exam Tip
Don’t confuse pencil lead with the metal lead – they have nothing in common. Pencil lead is actually graphite, and historical research suggests that in the past, lead miners sometimes confused the mineral galena (lead sulfide) with graphite; since the two looked similar they termed both minerals ‘lead’.The word graphite derives from the Latin word ‘grapho’ meaning ‘I write’, so it is a well named mineral!
转载自savemyexam

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








