- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.7.3 Simple Molecular Structures
Edexcel IGCSE Chemistry 复习笔记 1.7.3 Simple Molecular Structures
Simple Molecular Structures
- Simple molecular structures have covalent bonds joining the atoms together, but intermolecular forces that act between neighbouring molecules
- They have low melting and boiling points as there are only weak intermolecular forces acting between the molecules
- These forces are very weak when compared to the covalent bonds and so most small molecules are either gases or liquids at room temperature
- Often the liquids are volatile
- As the molecules increase in size the intermolecular forces also increase as there are more electrons available
- This causes the melting and boiling points to increase
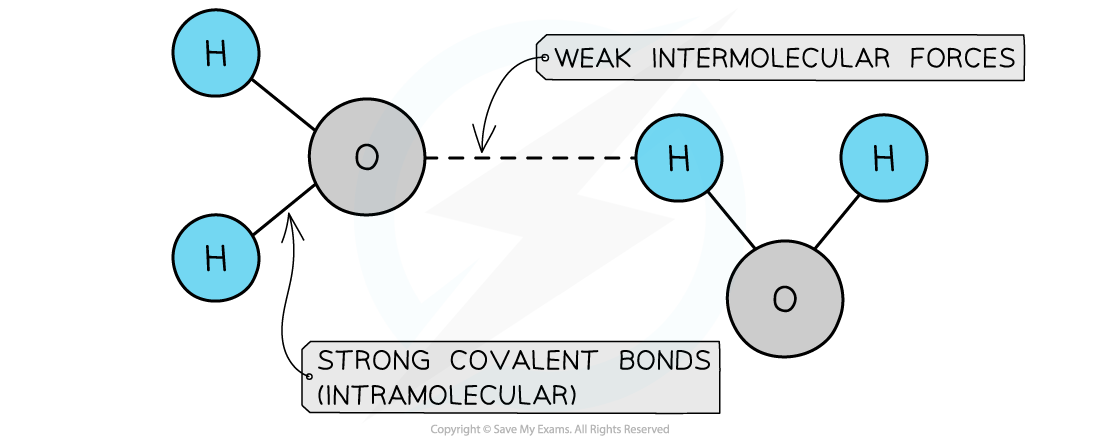
Covalent bonds are strong but intermolecular forces are weak
Exam Tip
The atoms within covalent molecules are held together by covalent bonds while the molecules in a covalent substance are attracted to each other by intermolecular forces.
Melting & Boiling Point Patterns
Melting and Boiling Point of Simple Compounds in Relation to Molecular Mass
- As the relative molecular mass of a substance increases, the melting and boiling point will increase as well
- An increase in the relative molecular mass of a substance means that there are more electrons in the structure, so there are more intermolecular forces of attraction that need to be overcome when a substance changes state
- So larger amounts of heat energy are needed to overcome these forces, causing the compound to have a higher melting and boiling point
- The family of organic molecules called alkanes show a clear increase in boiling point as the size of the molecule increases
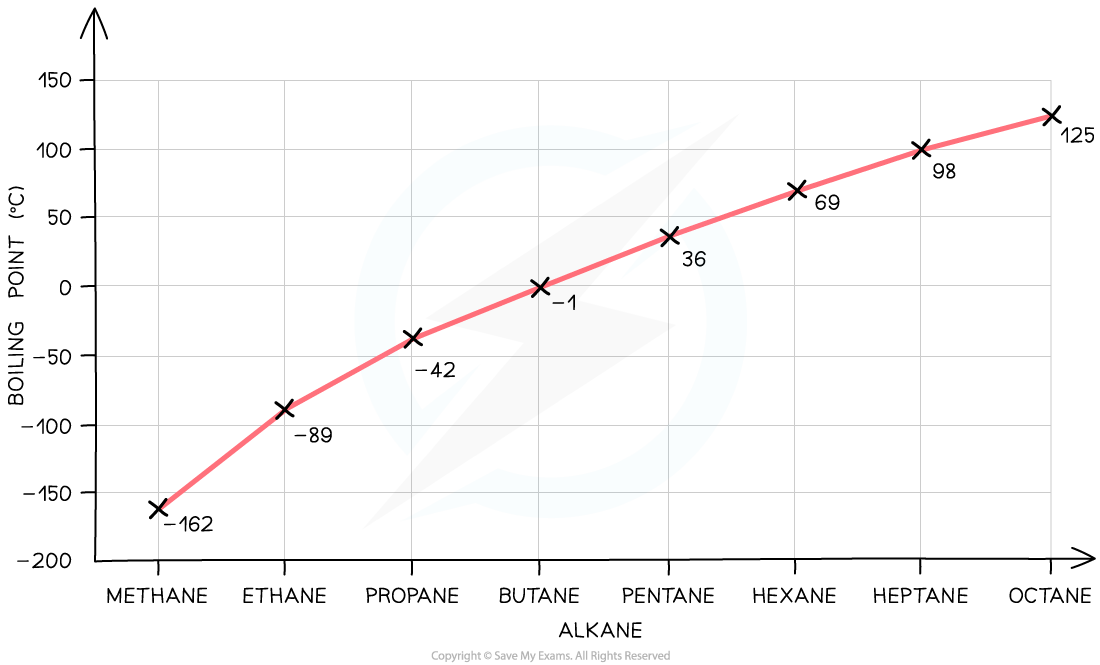
Graph showing the increase in boiling point as the molecular size increases
Conductivity & Covalent Compounds
- They are poor conductors of electricity as there are no free ions or electrons to carry the charge
- Most covalent compounds do not conduct at all in the solid state and are thus insulators
- Common insulators include the plastic coating around household electrical wiring, rubber and wood
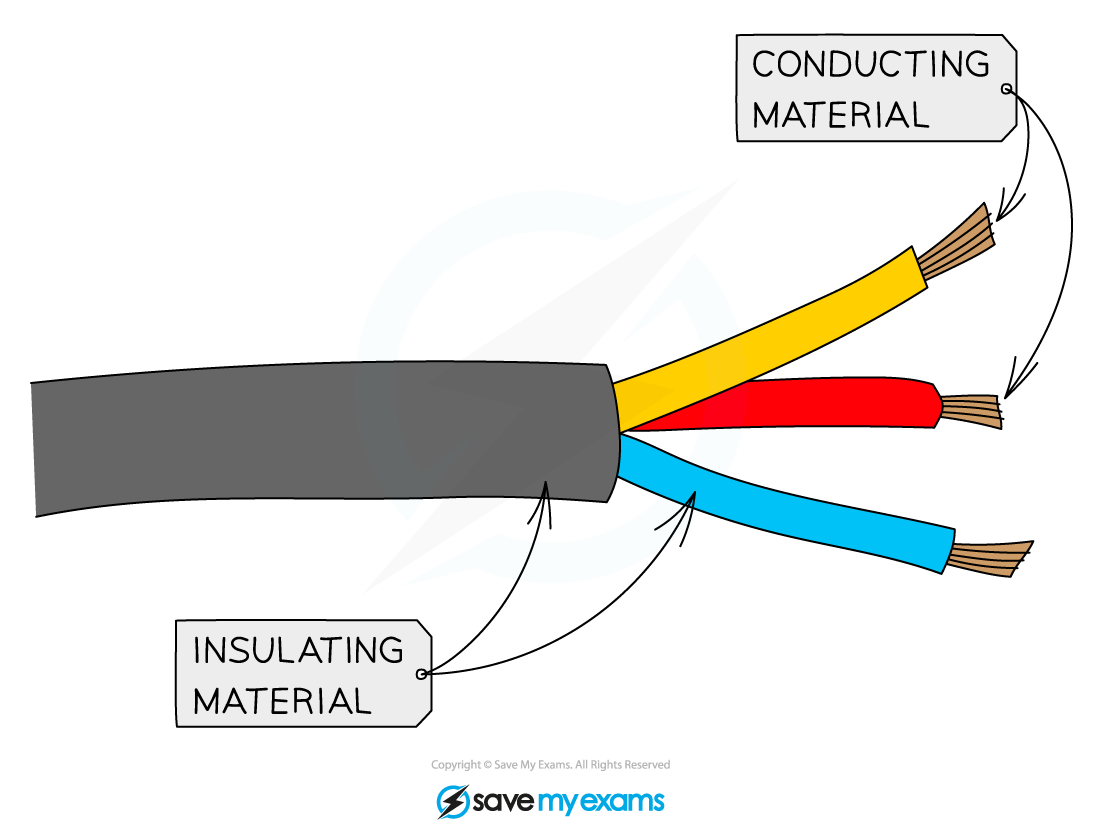
The plastic coating around electrical wires is made from covalent substances that do not allow a flow of charge
Exam Tip
When a covalent molecule melts or boils the covalent bonds do not break, only the intermolecular forces. If you think about it, when you boil a kettle full of water you are not generating large volumes of hydrogen and oxygen gas in your kitchen – this might give you an interesting unwanted chemical reaction ! Boom !
转载自savemyexam


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








