- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds: Bonds, Structure & Properties
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds: Bonds, Structure & Properties
Ionic bonding
- The positive and negative charges are held together by the strong electrostatic forces of attraction between oppositely charged ions
- This is what holds ionic compounds together
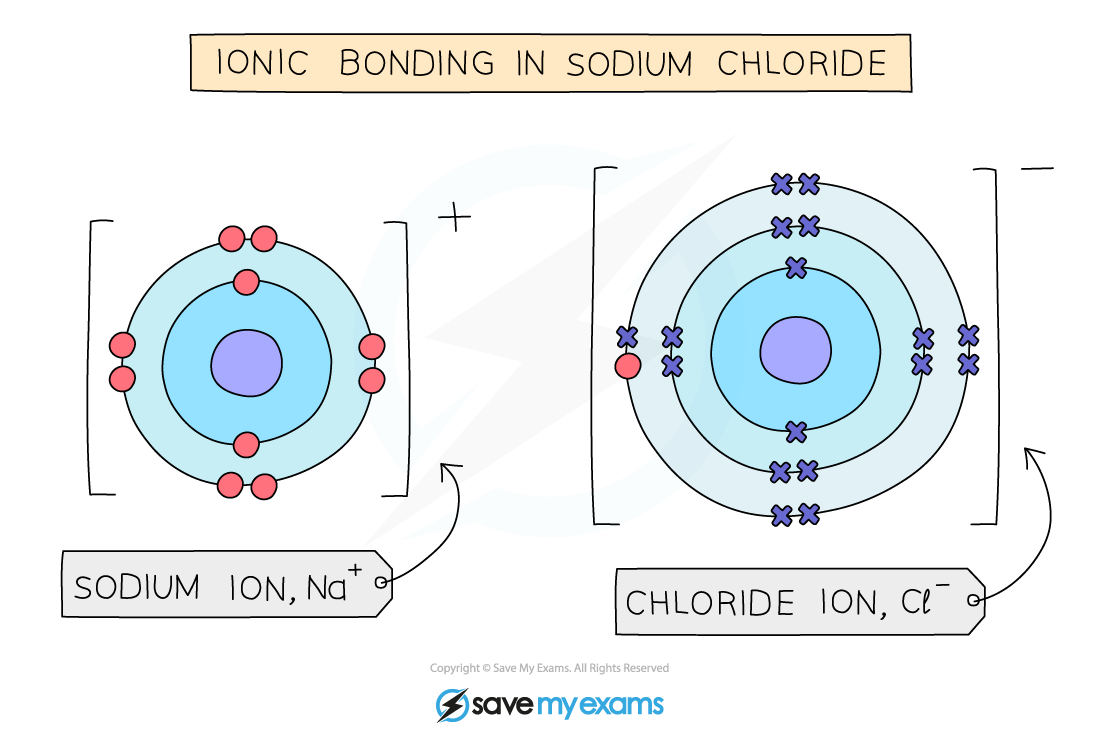
Electrostatic forces hold the ions together in sodium chloride
Giant Ionic Lattices
- Ionic compounds are made of charged particles called ions which form a giant lattice structure
- Ionic substances have high melting and boiling points due to the presence of strong electrostatic forces acting between the oppositely charged ions
- These forces act in all directions and a lot of energy is required to overcome them
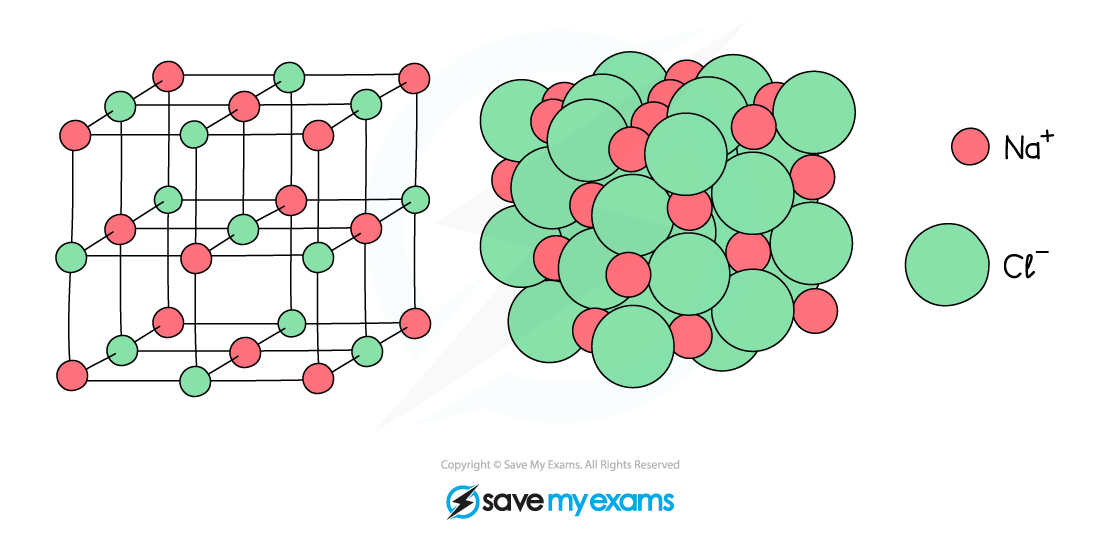
Strong electrostatic forces act in all directions in an ionic solid such as sodium chloride
- Ionic compounds are usually solid at room temperature and are non-volatile
- They are usually water soluble as both ionic compounds and water are polar substances
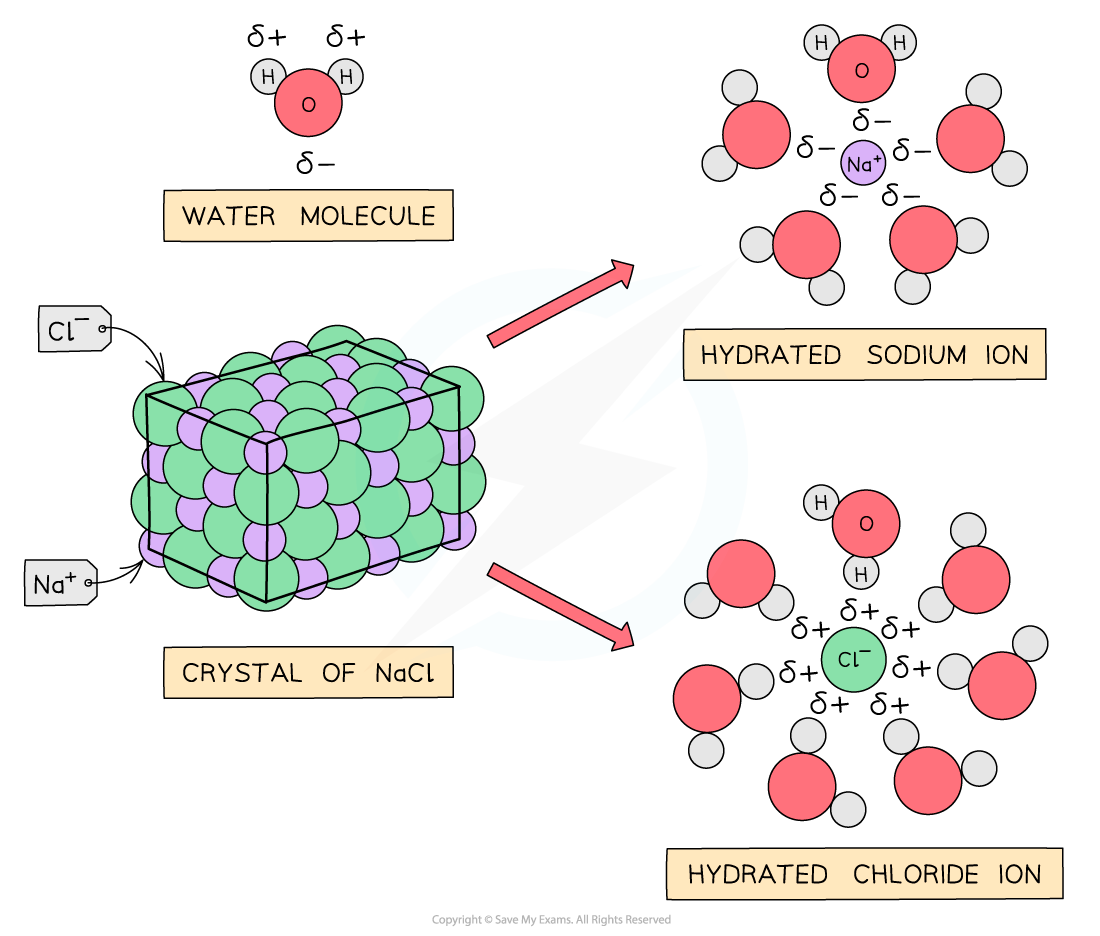
Ionic compounds are soluble in water because the ions are easily hydrated by polar water molecules
Exam Tip
Ions with higher charge have stronger electrostatic forces and will thus have higher melting and boiling points.
Conductivity & Ionic Compounds
- For electrical current to flow there must be present freely moving charged particles such as electrons or ions
- Ionic compounds can conduct electricity in the molten state or in solution as they have ions that can move and carry charge
- They cannot conduct electricity in the solid state as the ions are in fixed positions within the lattice and are unable to move
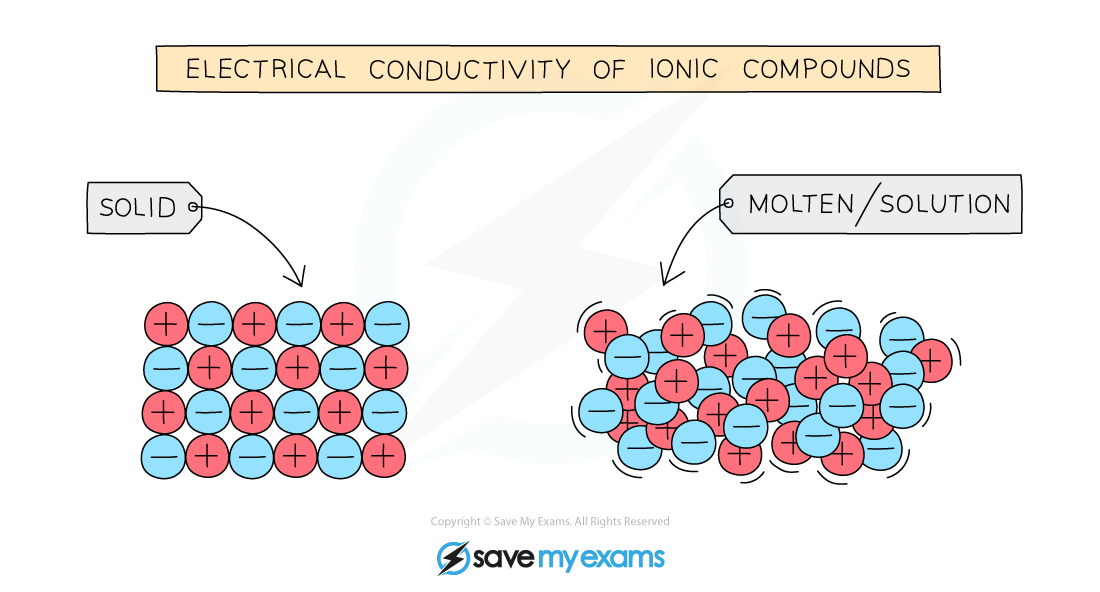
Molten or aqueous particles move and conduct electricity but cannot in the solid state
Exam Tip
Remember that in ionic lattice structures, positively charged and negatively charged ions are arranged in an alternating pattern.
转载自savemyexam


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








