- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.5.1 Word & Chemical Equations
Edexcel IGCSE Chemistry 复习笔记 1.5.1 Word & Chemical Equations
Writing Equations
Nothing created - nothing destroyed
-
- However, the same atoms are always present before and after reaction
- They have just joined up in different ways
- Atoms cannot be created or destroyed, so if they exist in the reactants then they absolutely must be in the products!New substances are made during chemical reactions
- Because of this the total mass of reactants is always equal to the total mass of products
- This idea is known as the Law of Conservation of Mass
Conservation of Mass
- The Law of Conservation of Mass enables us to balance chemical equations, since no atoms can be lost or created
-
- Write word equations for reactions outlined in these notes
- Write formulae and balanced chemical equations for the reactions in these notesYou should be able to:
Word Equations
- These show the reactants and products of a chemical reaction using their full chemical names
- The reactants are those substances on the left-hand side of the arrow and can be thought of as the chemical ingredients of the reaction
- They react with each other and form new substances
- The products are the new substances which are on the right-hand side of the arrow
- The arrow (which is spoken as “goes to” or “produces”) implies the conversion of reactants into products
- Reaction conditions or the name of a catalyst (a substance added to make a reaction go faster) can be written above the arrow
- An example is the reaction of sodium hydroxide (a base) and hydrochloric acid produces sodium chloride (common table salt) and water:
sodium hydroxide + hydrochloric acid ⟶ sodium chloride + water
Representing reactions as equations
- Chemical equations use the chemical symbols of each reactant and product
- When balancing equations, there has to be the same number of atoms of each element on either side of the equation in accordance with the Law of Conservation of Mass
- A symbol equation uses the formulae of the reactants and products to show what happens in a chemical reaction
- A symbol equation must be balanced to give the correct ratio of reactants and products:
S + O2 → SO2
- This equation shows that one atom of sulfur (S) reacts with one molecule of oxygen (O2) to make one molecule of sulfur dioxide (SO2)
- The following non-metals must be written as molecules: H2, N2, O2, F2, Cl2, Br2 and I2
- To balance an equation you work across the equation from left to right, checking one element after another
- If there is a group of atoms, for example a nitrate group (NO3–) that has not changed from one side to the other, then count the whole group as one entity rather than counting the individual atoms
-
- Acid-base neutralisation reaction:Examples of chemical equations:
NaOH (aq) + HCl (aq) ⟶ NaCl (aq) + H2O (l)
-
- Redox reaction:
2Fe2O3 (aq) + 3C (s) ⟶ 4Fe (s) + 3CO2 (g)
- In each equation there are equal numbers of each atom on either side of the reaction arrow so the equations are balanced
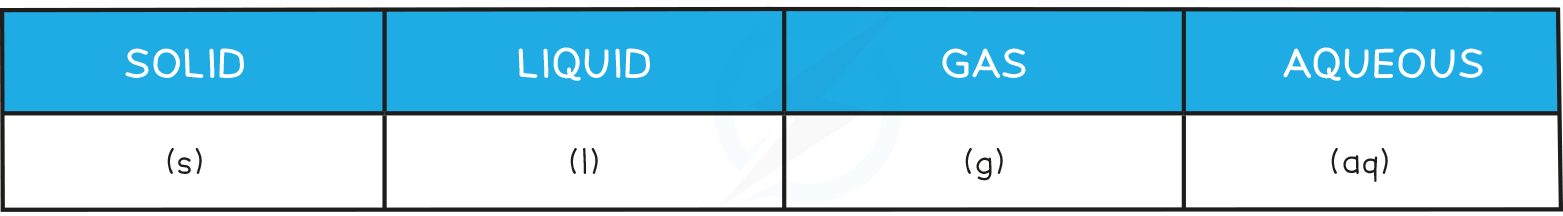
- Don't forget to add state symbols when writing balanced equations:
Balancing Equations
The best approach is to practice lot of examples of balancing equations
- By trial and error change the coefficients (multipliers) in front of the formulae, one by one checking the result on the other side
- Balance elements that appear on their own, last in the process
Worked Example
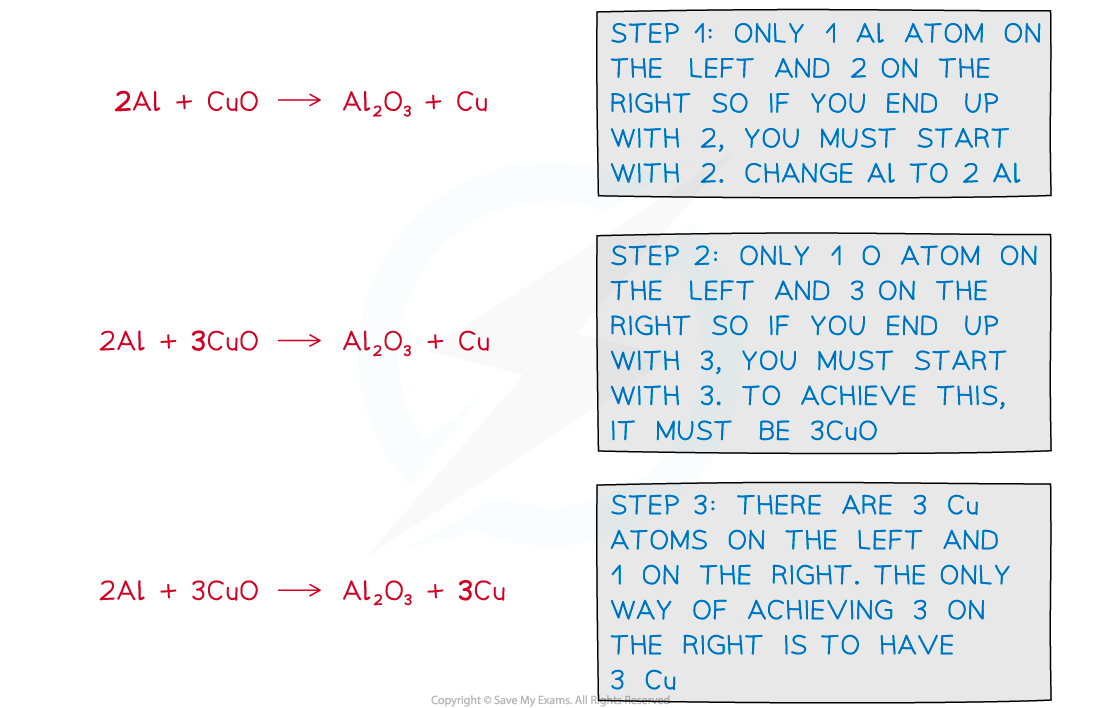
Example 1Balance the following equation:
aluminium + copper(II)oxide ⟶ aluminium oxide + copper
Unbalanced symbol equation:
Al + CuO ⟶ Al2O3 + Cu
Answer
Worked Example
Example 2
Balance the following equation:
magnesium oxide + nitric acid ⟶ magnesium nitrate + water
Unbalanced symbol equation:
MgO + HNO3 ⟶ Mg(NO3)2 + H2O
Answer

Exam Tip
Chemical equations do not contain an equals sign between the left and right-hand sides but are written with an arrow instead. The arrow means that the reactants have reacted together and formed the product(s).
转载自savemyexam

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








