- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.4.2 Electronic Configurations
Edexcel IGCSE Chemistry 复习笔记 1.4.2 Electronic Configurations
Deducing Electronic Configurations
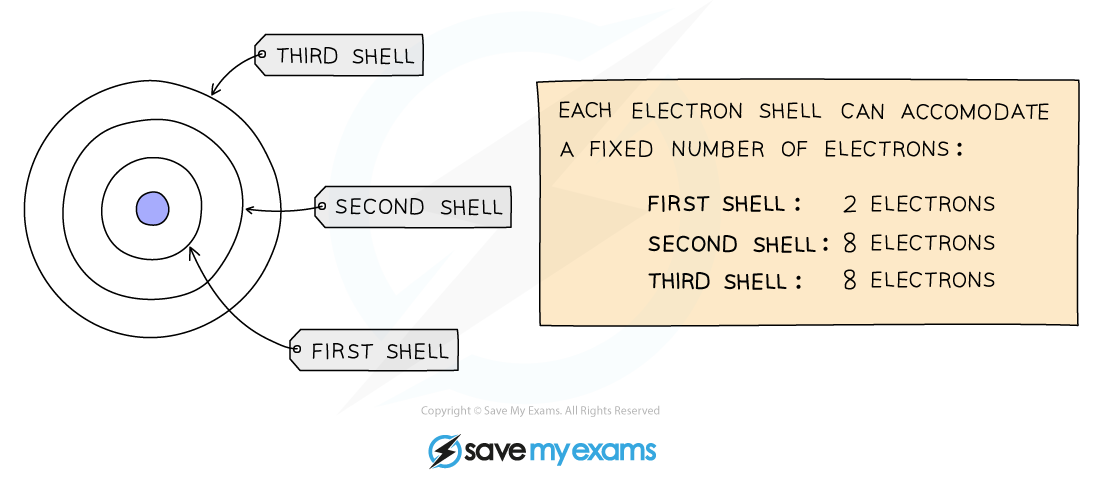
- We can represent the electronic structure of atoms using electron shell diagrams
- Electrons orbit the nucleus in shells and each shell has a different amount of energy associated with it
- The further away from the nucleus, the more energy a shell has
- Electrons first occupy the shell closest to the nucleus which can hold a maximum of 2 electrons
- When a shell becomes full of electrons, additional electrons have to be added to the next shell
- The second shell and third shell can hold 8 electrons each
- The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons
- In most atoms, the outermost shell is not full and therefore these atoms react with other atoms in order to achieve a full outer shell of electrons (which would make them more stable)
- In some cases, atoms lose electrons to entirely empty this shell so that the next shell below becomes a (full) outer shell
Deducing electron configuration
- You should be able to write the electron configuration for the first twenty elements
Electronic Configuration of the First 20 Elements Table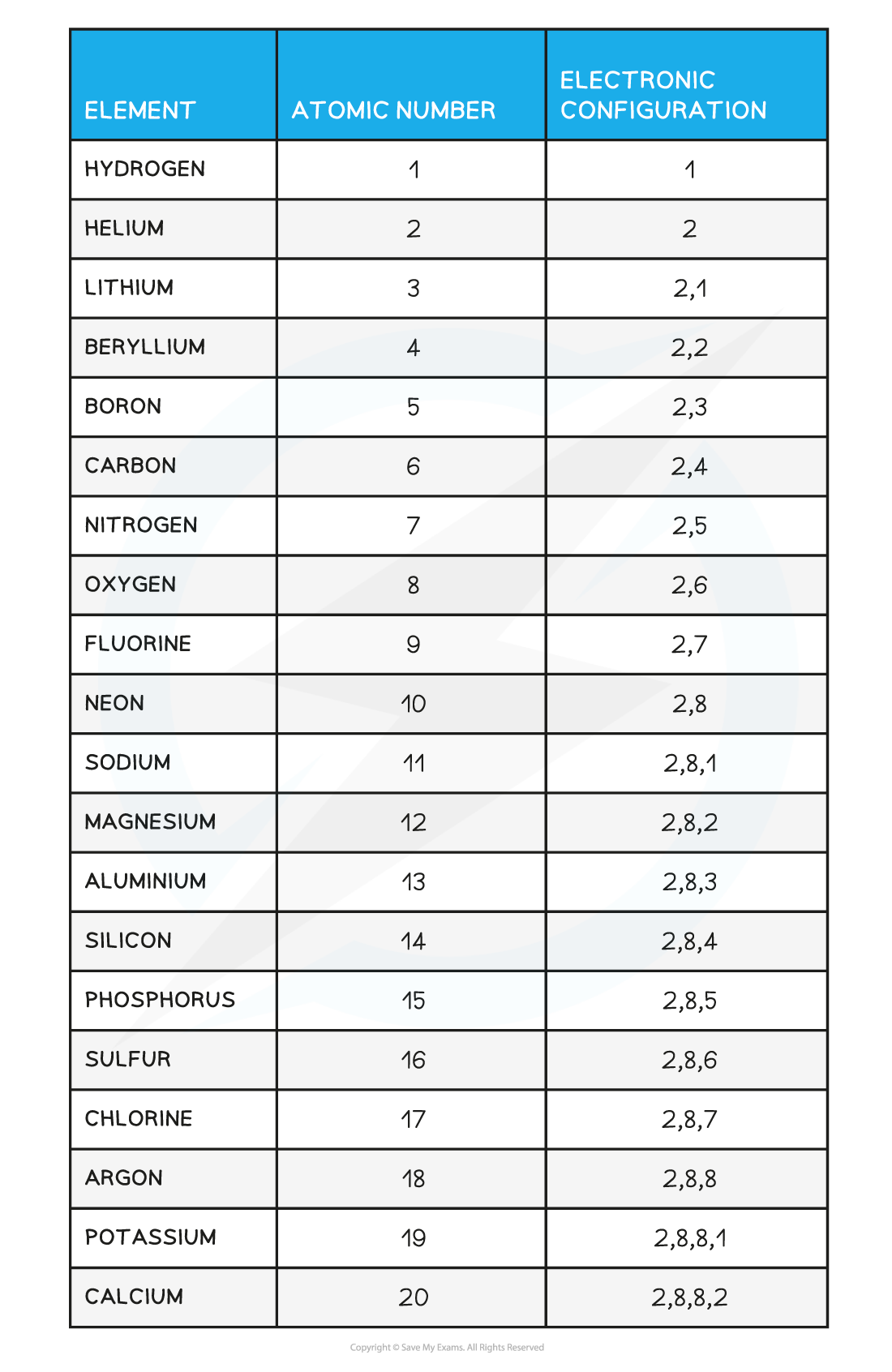
Note: Although the third shell can hold up to 18 electrons, the filling of the shells follows a more complicated pattern after potassium and calcium. For these two elements, the third shell holds 8 and the remaining electrons (for reasons of stability) occupy the fourth shell first before filling the third shell
Exam Tip
You should be able to represent the first 20 elements using either electron shell diagrams or written electronic configuration.
Electronic Configurations & the Periodic Table
Electronic configuration: The arrangement of electrons into shells for an atom (e.g. electronic configuration of carbon is 2 . 4)
Electronic configuration and position in periodic table
- The number of notations in the electronic configuration will show the number of shells of electrons the atom has, showing the period
- The last notation shows the number of outer electrons the atom has, showing the group
Example: Electronic configuration of chlorine:
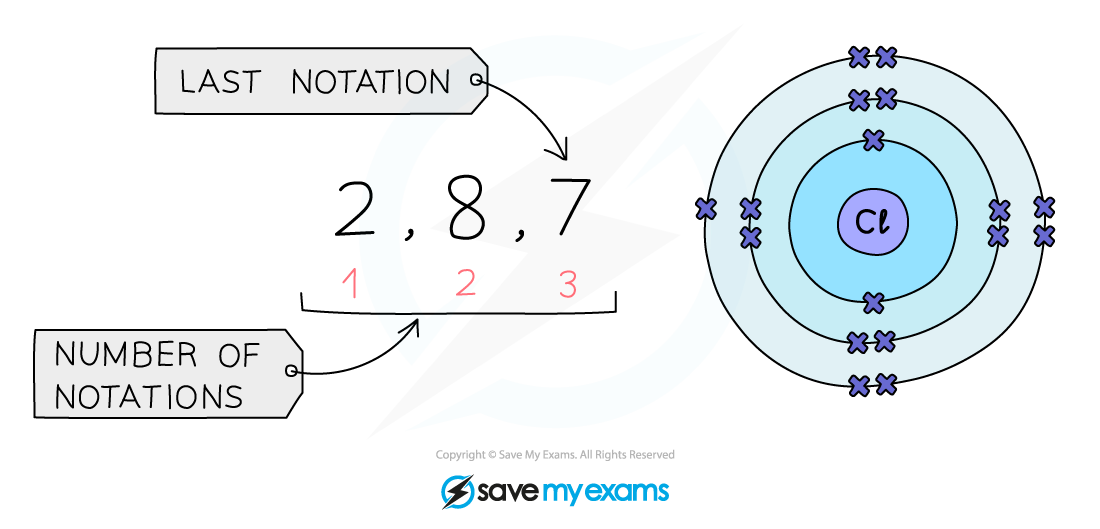
Shorthand electronic configuration
The position of chlorine on the periodic table
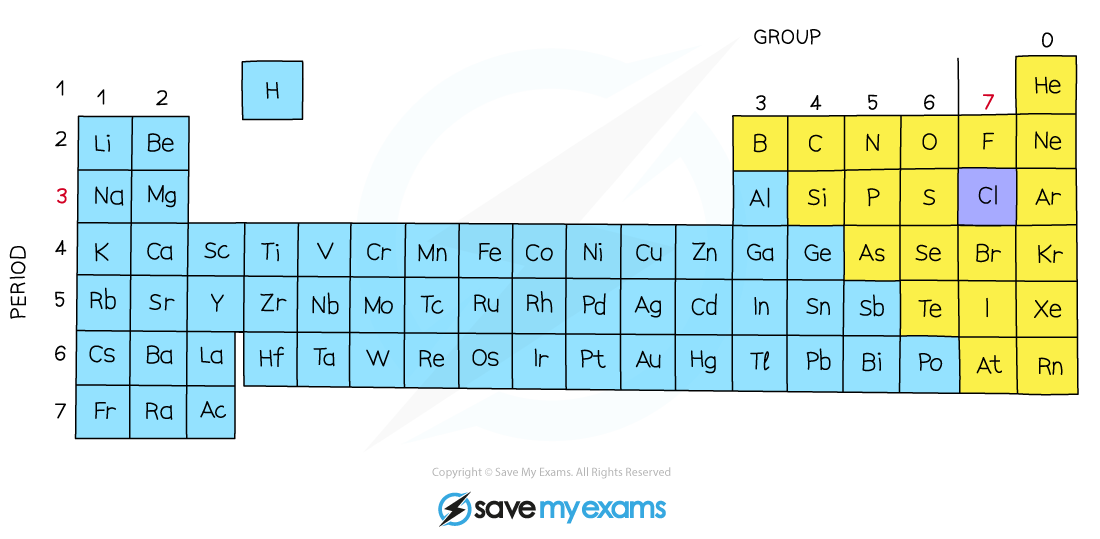
The position of chlorine in the periodic table
Exam Tip
All of the shells up to the outer shell will be full. Electron transfer occurs with electrons from the outer shell only.You can use the term ‘shell’ or ‘energy level’ to describe the space that electrons occupy.
转载自savemyexam

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








