- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.3.2 Calculate Relative Atomic Mass
Edexcel IGCSE Chemistry 复习笔记 1.3.2 Calculate Relative Atomic Mass
Calculate Relative Atomic Mass
- The relative atomic mass of each element is calculated from the mass number and relative abundances of all the isotopes of a particular element
- The steps below are to calculate the relative atomic mass
- Start by finding out the mass of 100 atoms, then divide the result by 100 to get the Ar
- The top line of the equation can be extended to include the number of different isotopes of a particular element present, so, if there were 3 isotopes present then the top line of the equation would read:
Worked Example
The table shows information about the Isotopes in a sample of rubidium with 72% 85Rb and 28% 87Rb
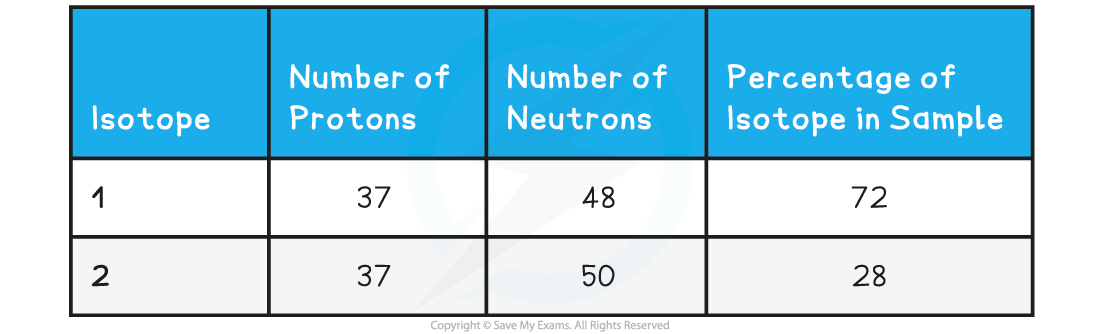
Use information from the table to calculate the relative atomic mass of this sample of Rubidium. Give your answer to one decimal place:

Use information from the table to calculate the relative atomic mass of this sample of Rubidium. Give your answer to one decimal place:
Answer 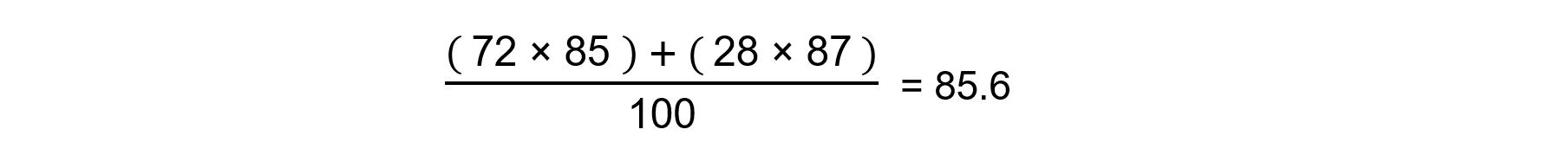
Relative Atomic Mass = 85.6
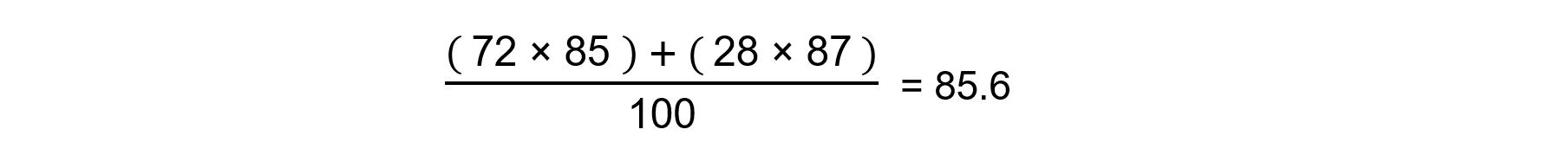
Relative Atomic Mass = 85.6
Exam Tip
Isotopes are easy to recognize from their notation as they have the same symbol but different mass numbers. For example, the two stable isotopes of copper are 63Cu and 65Cu
转载自savemyexam

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









