- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记7.4.1 Coulomb's Law
Coulomb's Law
- All charged particles produce an electric field around it
- This field exerts a force on any other charged particle within range
- The electrostatic force between two charges is defined by Coulomb’s Law
- Recall that the charge of a uniform spherical conductor can be considered as a point charge at its centre
- Coulomb’s Law states that:
The electrostatic force between two point charges is proportional to the product of the charges and inversely proportional to the square of their separation
- The Coulomb equation is defined as:
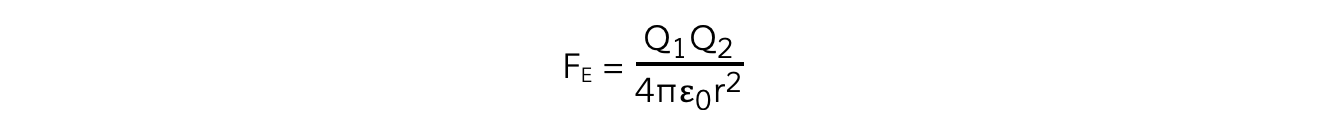

The electrostatic force between two charges is defined by Coulomb’s Law
- Where:
- FE = electrostatic force between two charges (N)
- Q1 and Q2 = two point charges (C)
- ε0 = permittivity of free space
- r = distance between the centre of the charges (m)
- The 1/r2 relation is called the inverse square law
- This means that when the separation of two charges doubles, the electrostatic force between them reduces by (½)2 = ¼
- ε0 is a physical constant used to show the capability of a vacuum to permit electric fields
- If there is a positive and negative charge, then the electrostatic force is negative
- This can be interpreted as an attractive force
- If the charges are the same, the electrostatic force is positive
- This can be interpreted as a repulsive force
- Since uniformly charged spheres can be considered as point charges, Coulomb’s law can be applied to find the electrostatic force between them as long as the separation is taken from the centre of both spheres
Worked Example
An alpha particle is situated 2.0 mm away from a gold nucleus in a vacuum. Assuming them to be point charges, calculate the magnitude of the electrostatic force acting on each of the charges.
Atomic number of helium = 2
Atomic number of gold = 79
Charge of an electron = 1.60 × 10-19 C
Step 1: Write down the known quantities
-
- Distance, r = 2.0 mm =2.0 × 10-3 m
The charge of one proton = +1.60 × 10-19 C
An alpha particle (helium nucleus) has 2 protons
-
- Charge of alpha particle, Q1 = 2 × 1.60 × 10-19 = +3.2 × 10-19 C
The gold nucleus has 79 protons
-
- Charge of gold nucleus, Q2 = 79 × 1.60 × 10-19 = +1.264 × 10-17 C
Step 2: The electrostatic force between two point charges is given by Coulomb’s Law
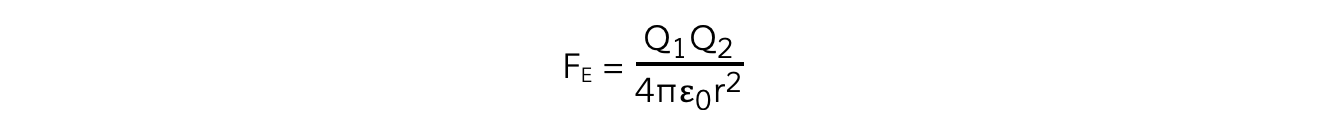
Step 3: Substitute values into Coulomb's Law
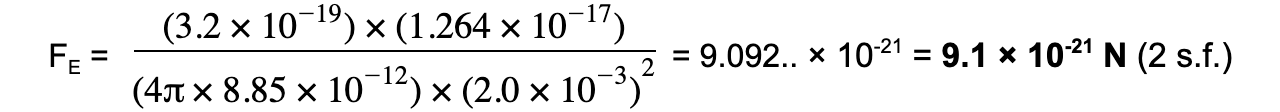
Approximations of Coulomb's Law
- When calculating the force between two charges, air is treated as a vacuum
- This is why ε0, the permittivity of free space is used
- For a point outside a spherical conductor, the charge of the sphere may be considered to be a point charge at its centre
- A uniform spherical conductor is one where its charge is distributed evenly
- The electric field lines around a spherical conductor are therefore identical to those around a point charge
- An example of a spherical conductor is a charged sphere
- The field lines are radial and their direction depends on the charge of the sphere
- If the spherical conductor is positively charged, the field lines are directed away from the centre of the sphere
- If the spherical conductor is negatively charged, the field lines are directed towards the centre of the sphere
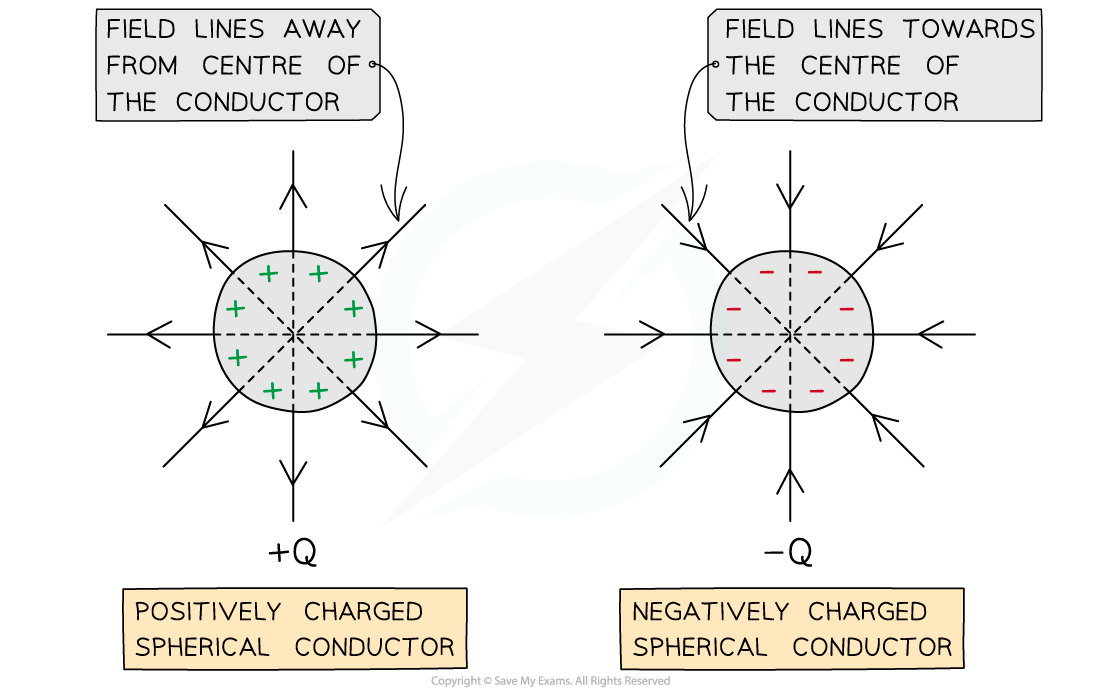
Electric field lines around a uniform spherical conductor are identical to those on a point charge
Exam Tip
You may have noticed that the electric fields share many similarities to the gravitational fields. The main difference being the gravitational force is always attractive, whilst electrostatic forces can be attractive or repulsive.You should make a list of all the similarities and differences you can find, as this could come up in an exam question.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









