- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
Edexcel IGCSE Chemistry 复习笔记 1.1.4 Solubility
Edexcel IGCSE Chemistry 复习笔记 1.1.4 Solubility
Solubility
- Solubility is a measurement of how much of a substance will dissolve in a given volume of a liquid
- The liquid is called the solvent
- The solubility of a gas depends on pressure and temperature
- Different substances have different solubilities
- Solubility can be expressed in g per 100 g of solvent
- Solubility of solids is affected by temperature
- As temperature increases, solids usually become more soluble
- Solubility of gases is affected by temperature and pressure; in general:
- As pressure increases, gases become more soluble
- As temperature increases, gases become less soluble
Solubility Curves
- Solubility graphs or curves represent solubility in g per 100 g of water plotted against temperature
- To plot a solubility curve, the maximum mass of solvent that can be dissolved in 100 g of water before a saturated solution is formed, is determined at a series of different temperatures
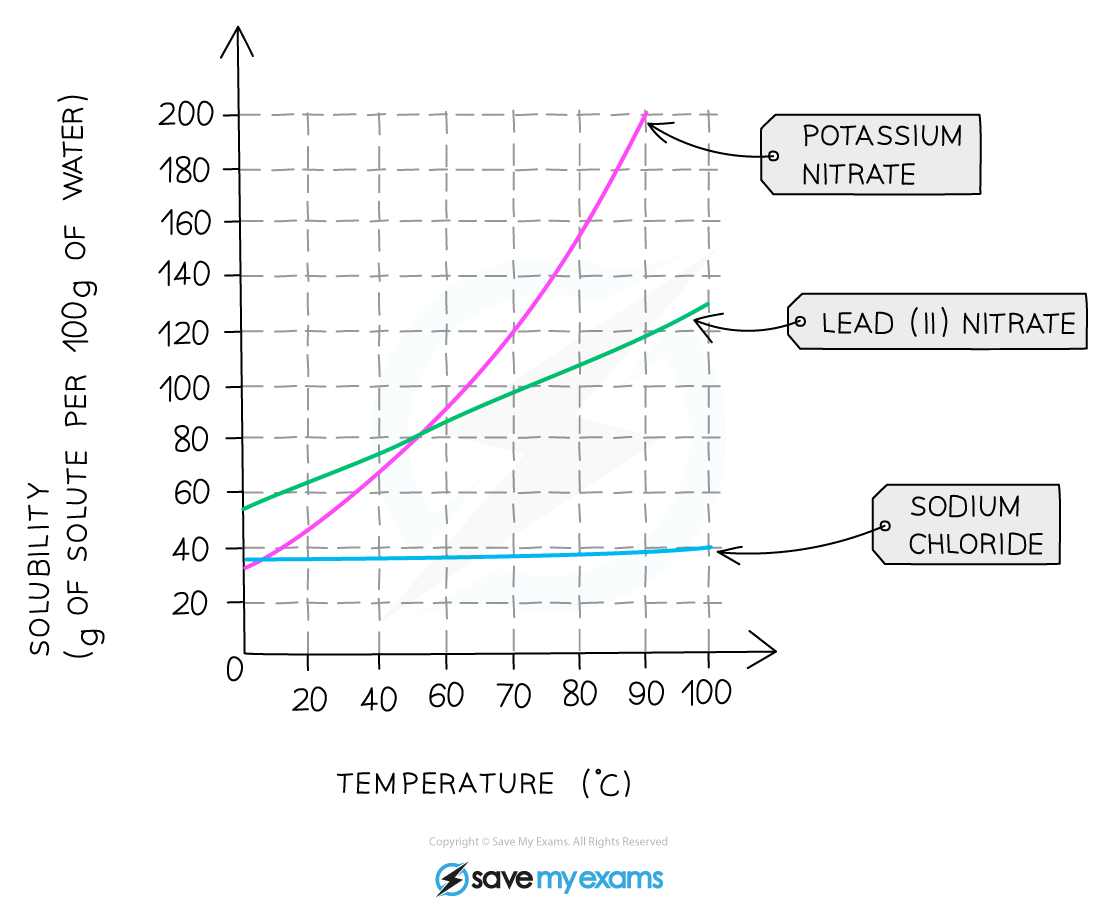
Solubility curve for three salts. While the solubility of most salts increases with temperature, sodium chloride, or common salt, hardly changes at all
Worked Example
Use the solubility curve to answer these questions:
- Determine how much potassium nitrate will dissolve in 20 g of water at 40 °C?
- 200 cm3 of saturated lead(II)nitrate solution was prepared at a temperature of 90 °C. What mass of lead(II)nitrate crystals form if the solution was cooled to 20 °C?
Answers
Problem 1
At 40 °C the solubility is 68 g per 100 g of water
So scaling, 68 x (20 / 100) = 13.6 g of potassium nitrate will dissolve in 20 g of water
Problem 2
Solubility of lead(II) nitrate at 90 oC is 118 g / 100 g water, and 64 g / 100 g water at 20 °C.
Therefore for mass of crystals formed = 118 – 64 = 54 g (for 100 cm3 of solution).
However, 200 cm3 of solution was prepared,
So total mass of lead(II) nitrate crystallised = 2 x 54 = 108 g
Exam Tip
As temperature increases, solids usually become more soluble and gases become less soluble.
转载自savemyexam

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









