- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记6.6.3 Average Molecular Kinetic Energy
Average Molecular Kinetic Energy
- An important property of molecules in a gas is their average kinetic energy
- This can be deduced from the ideal gas equations relating pressure, volume, temperature and speed
- Recall the ideal gas equation:
pV = NkT
- Also, recall the equation linking pressure and mean square speed of the molecules:
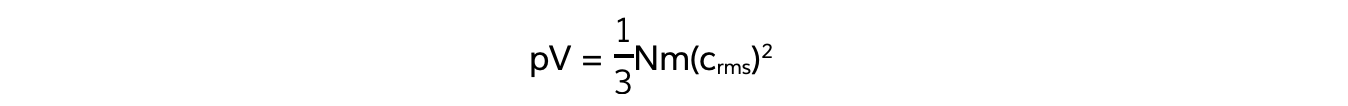
- The left-hand side of both equations are equal (pV)
- This means the right-hand sides are also equal:
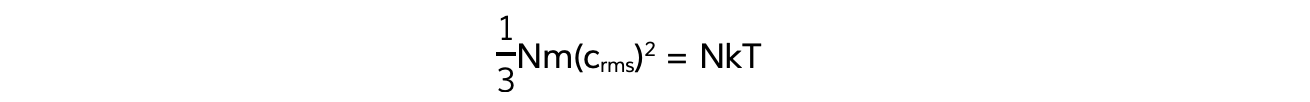
- N will cancel out on both sides and multiplying by 3 on both sides too obtains the equation:
m(crms)2 = 3kT
- Recall the familiar kinetic energy equation from mechanics:

- Instead of v2 for the velocity of one particle, (crms)2 is the average speed of all molecules
- Multiplying both sides of the equation by ½ obtains the average molecular kinetic energy of the molecules of an ideal gas:
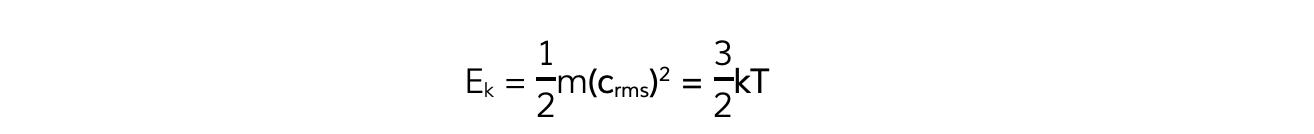
- Where:
- Ek = kinetic energy of a molecule (J)
- m = mass of one molecule (kg)
- (crms)2 = mean square speed of a molecule (m2 s-2)
- k = Boltzmann constant
- T = temperature of the gas (K)
- Note: this is the average kinetic energy for only one molecule of the gas
- A key feature of this equation is that the mean kinetic energy of an ideal gas molecule is proportional to its thermodynamic temperature
Ek ∝ T
- The Boltzmann constant k can be replaced with
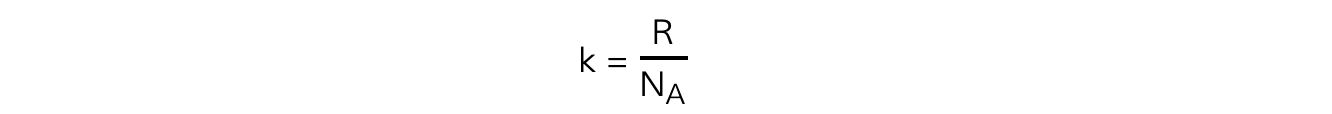
- Substituting this into the average molecular kinetic energy equation means it can also be written as:
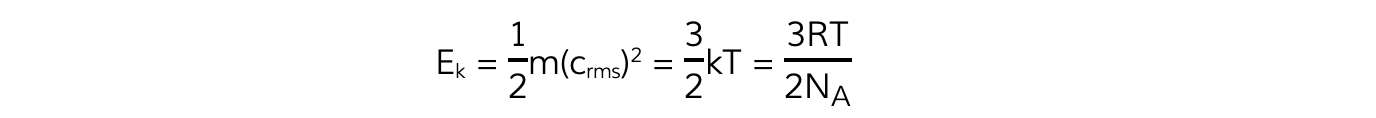
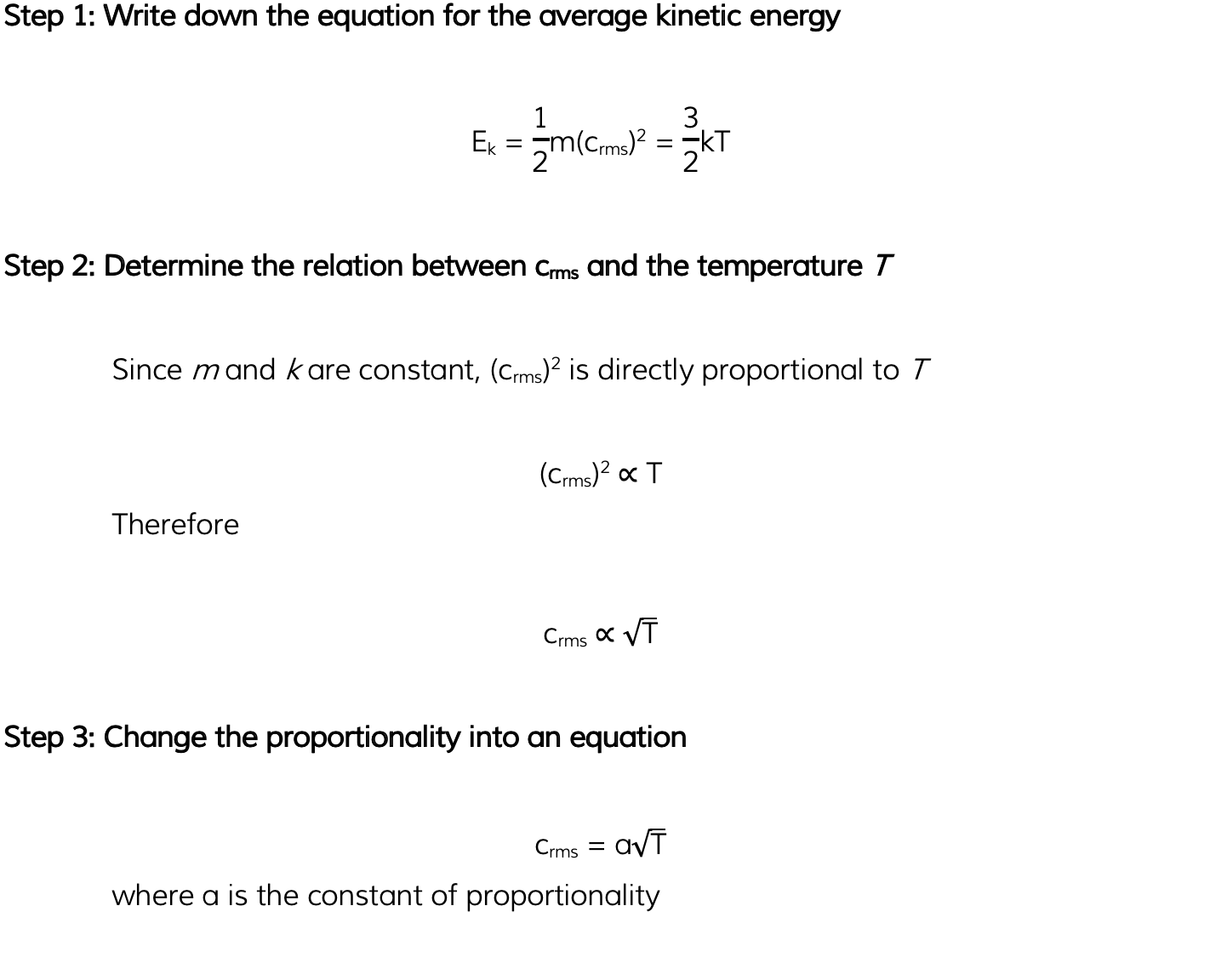
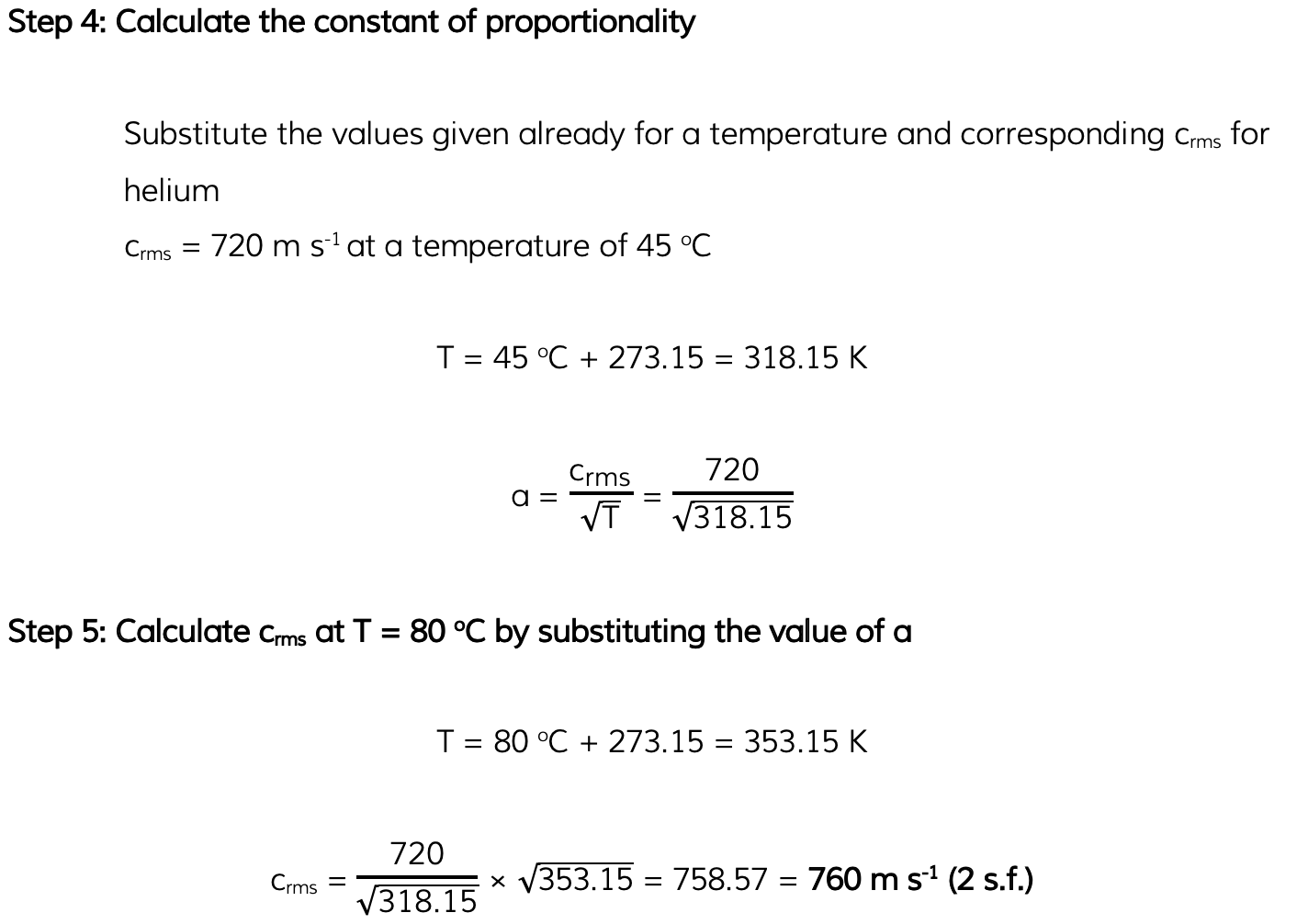
Worked Example
Helium can be treated as an ideal gas.Helium molecules have a root-mean-square (r.m.s.) speed of 730 m s-1 at a temperature of 45 °C.Calculate the r.m.s. speed of the molecules at a temperature of 80 °C.
Exam Tip
Keep in mind this particular equation for kinetic energy is only for one molecule in the gas. If you want to find the kinetic energy for all the molecules, remember to multiply by N, the total number of molecules.You can remember the equation through the rhyme ‘Average K.E is three-halves kT’.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








