- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记6.4.4 Latent Heat Capacity
Latent Heat Capacity
- Energy is required to change the state of a substance
- Examples of changes of state are:
- Melting = solid to liquid
- Evaporation / vaporisation / boiling = liquid to gas
- Sublimation = solid to gas
- Freezing = liquid to solid
- Condensation = gas to liquid
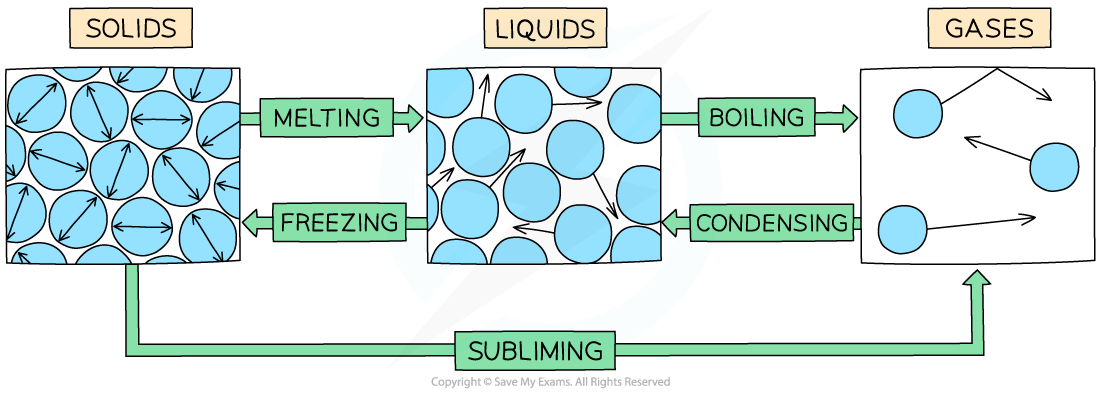
The example of changes of state between solids, liquids and gases
- When a substance changes state, there is no temperature change
- The energy supplied to change the state is called the latent heat and is defined as:
The thermal energy required to change the state of 1 kg of mass of a substance without any change of temperature
- There are two types of latent heat:
- Specific latent heat of fusion (melting)
- Specific latent heat of vaporisation (boiling)
- The larger the mass of the substance, the more energy will be required to change its state. Hence why specific latent heat is defined by 1 kg
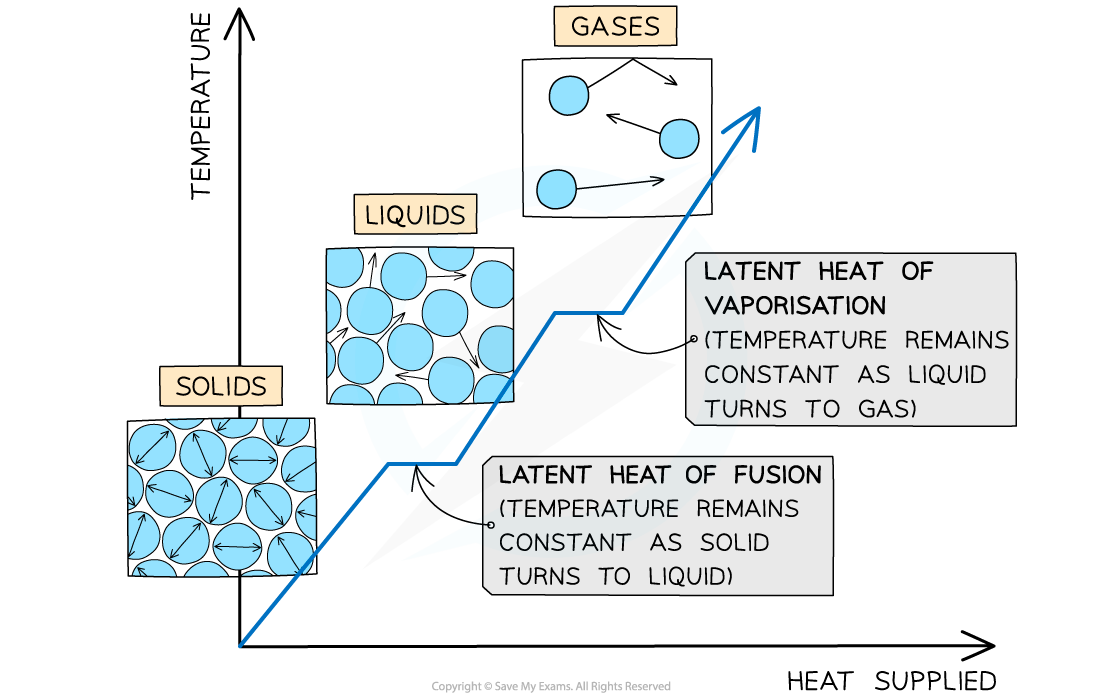
The changes of state with heat supplied against temperature. There is no change in temperature during changes of state
- The horizontal line of the latent heat of fusion represents melting (if heat is supplied) or freezing (if heat is removed)
- The horizontal line of the latent heat of vaporisation represents evaporation (if heat is supplied) or condensation (if heat is removed)
- The specific latent heat of fusion is defined as:
The thermal energy required to convert 1 kg of solid to liquid with no change in temperature
- Latent heat of fusion applies to:
- Melting a solid
- Freezing a liquid
- The specific latent heat of vaporisation is defined as:
The thermal energy required to convert 1 kg of liquid to gas with no change in temperature
- Latent heat of vaporisation applies to:
- Vaporising a liquid
- Condensing a gas
Calculating Specific Latent Heat
- The amount of energy Q required to melt or vaporise a mass of m with latent heat L is:
Q = mL
- Where:
- Q = amount of thermal energy to change the state (J)
- m = mass of the substance changing state (kg)
- L = latent heat of fusion or vaporisation (J kg-1)
- The values of latent heat for water are:
- Specific latent heat of fusion = 330 kJ kg-1
- Specific latent heat of vaporisation = 2.26 MJ kg-1
- Therefore, evaporating 1 kg of water requires roughly seven times more energy than melting the same amount of ice to form water
- The reason for this is to do with intermolecular forces:
- When ice melts: energy is required to just increase the molecule separation until they can flow freely over each other
- When water boils: energy is required to completely separate the molecules until there are no longer forces of attraction between the molecules, hence this requires much more energy. Vaporisation is also doing work against atmospheric pressure
- More energy has to be supplied to separate molecules than break a solid bond, which is why the latent heat of vaporisation of water is much greater than the specific latent heat of fusion of water
Worked Example
The energy needed to boil a mass of 530 g of a liquid is 0.6 MJ.Calculate the specific latent heat of the liquid and state whether it is the latent heat of vaporisation or fusion.
Step 1: Write the thermal energy required to change state equation
Q = mL
Step 2: Rearrange for latent heat
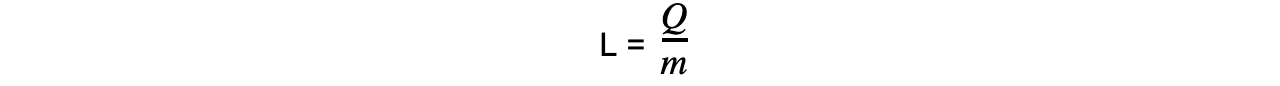
Step 3: Substitute in values
m = 530 g = 530 × 10-3 kg
Q = 0.6 MJ = 0.6 × 106 J
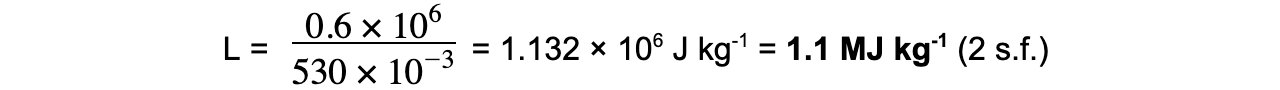
-
- L is the latent heat of vaporisation because the change in state is from liquid to gas (boiling)
Exam Tip
Use these reminders to help you remember which type of latent heat is being referred to:
- Latent heat of fusion = imagine ‘fusing’ the liquid molecules together to become a solid
- Latent heat of vaporisation = “water vapour” is steam, so imagine vaporising the liquid molecules into a gas
Remember to always include 'without a change in temperature', or words to that effect, within your definitions for latent heat to gain full marks.
Energy Transfers During Phase Changes
- When a substance is heated, the molecules are given more energy in the form of kinetic and potential energy
- During a change of state (or a phase change), the key points to remember are:
- There is no change in temperature
- The potential energies of the molecules change, but not their kinetic energies
- The potential energy of the molecules is due to their separation and intermolecular bonds
- Since they move further apart (evaporation) or closer together (condensation), their potential energy will change as a result of this
- The heat absorbed in melting and boiling causes the molecules to move further apart by overcoming the intermolecular forces of attraction
- The heat released in freezing and condensation allows the molecules to move closer together and the intermolecular forces of attraction become stronger
- This is because the kinetic energy is proportional to the temperature
- If there is no change in temperature, there must be no change in kinetic energy either
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









