- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记6.4.2 The First Law of Thermodynamics
The First Law of Thermodynamics
- The First Law of Thermodynamics, which is based on the principle of conservation of energy, states:
The internal energy of a system is increased when energy is transferred to it by heating or when work is done on it (and vice versa)
- This is important when thinking about the expansion or compression of a gas
- The First Law of Thermodynamics applies to all situations, not just for gases
- When a gas expands (its volume increases), work is done by the gas on the surroundings
- This decreases the internal energy of the gas
- When a gas is compressed (its volume decreases), work is done on the gas by the surroundings
- This increases the internal energy of the gas
- The 'gas' is sometimes referred to as the 'system'
- A gas can have work done on or by it when it is in a cylindrical container with a moveable piston
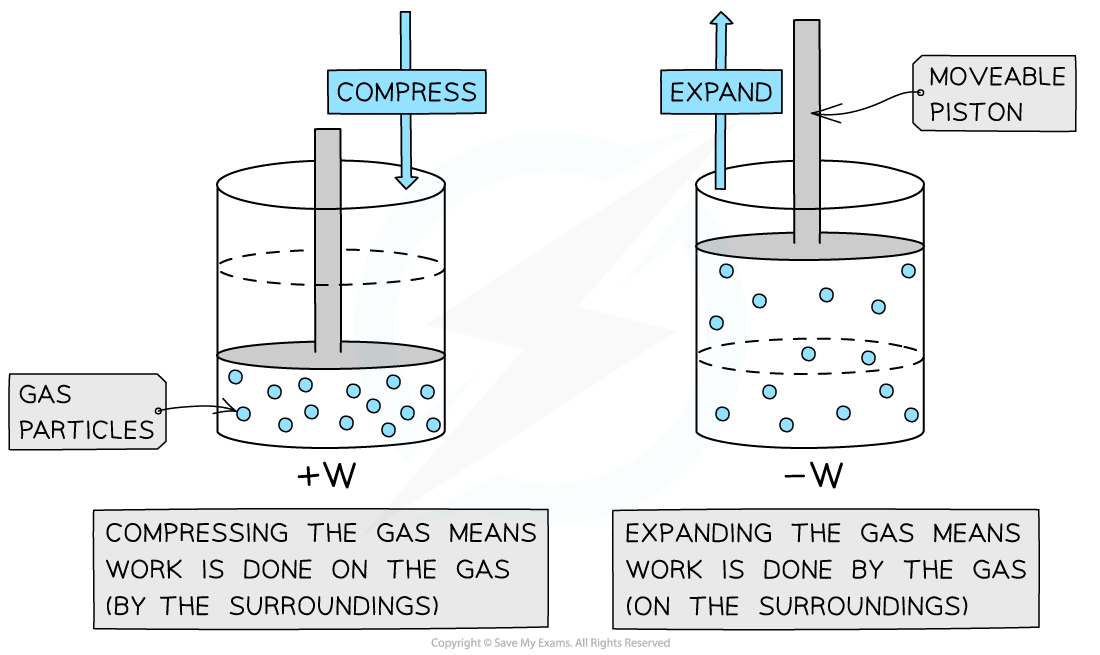
Work is done on the gas when it is compressed and by the gas when it expands
- When the piston moves down the cylinder, it compresses the gas molecules (work is done on the gas)
- The molecules are pushed closer together
- Therefore, they have higher kinetic energy as they move faster
- This increases the internal energy of the gas
- When the piston moves up the cylinder, it expands the gas molecules (work is done by the gas)
- The molecules are spread further apart
- Therefore, they have lower kinetic energy as they move slower
- This decreases the internal energy of the gas
- The same increase in internal energy can be achieved by not doing work (i.e. no expansion or contraction), but by heating the gas instead
- Increasing the temperature of the gas means the molecules move around faster
- They, therefore, have higher kinetic energy and increased internal energy
Exam Tip
Remember that the number of molecules in the container always remains the same whether the gas is expanding or contractingTry not to get too hung up on 'positive' and 'negative' work, this is more relevant for the 'Engineering Physics' option module. The important ideas to remember are when work is done by or on the gas (or system) and the ways in which internal energy changes.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









