- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记2.4.2 Threshold Frequency & Work Function
Threshold Frequency
- The photoelectric effect is the phenomena in which electrons are emitted from the surface of a metal upon the absorption of electromagnetic radiation
- Electrons removed from a metal in this manner are known as photoelectrons
- The photoelectric effect provides important evidence that light is quantised or carried in discrete packets
- This is shown by the fact each electron can absorb only a single photon
- This means only the frequencies of light above a threshold frequency will emit a photoelectron
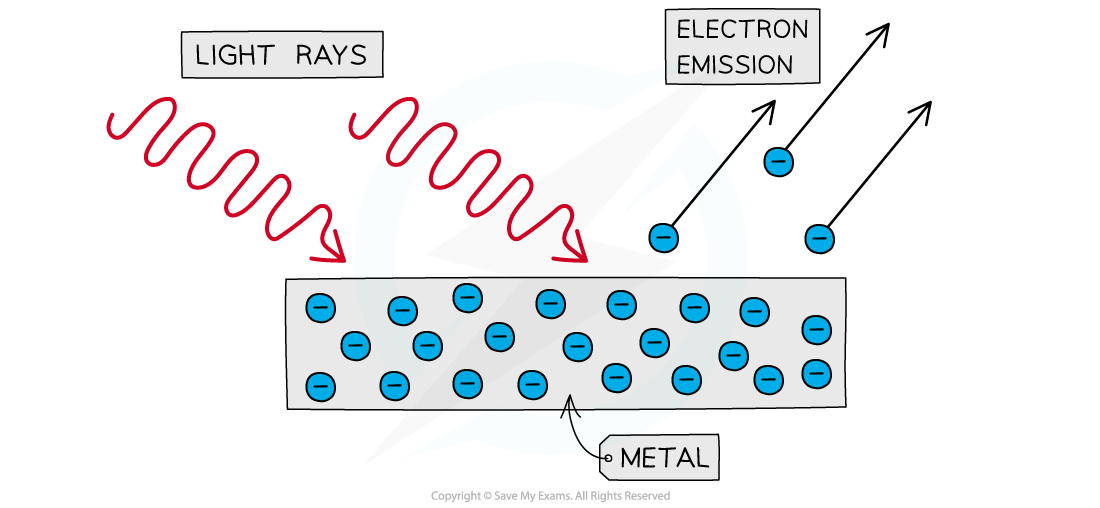
The photoelectric effect: photons of sufficient energy are able to liberate electrons from the surface of a metal
Threshold Frequency & Wavelength
- The threshold frequency is defined as:
The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal
- The threshold wavelength, related to threshold frequency by the wave equation, is defined as:
The longest wavelength of incident electromagnetic radiation that would remove a photoelectron from the surface of a metal
- The frequency and wavelength are related by the equation

- Since photons are particles of light, v = c (speed of light)
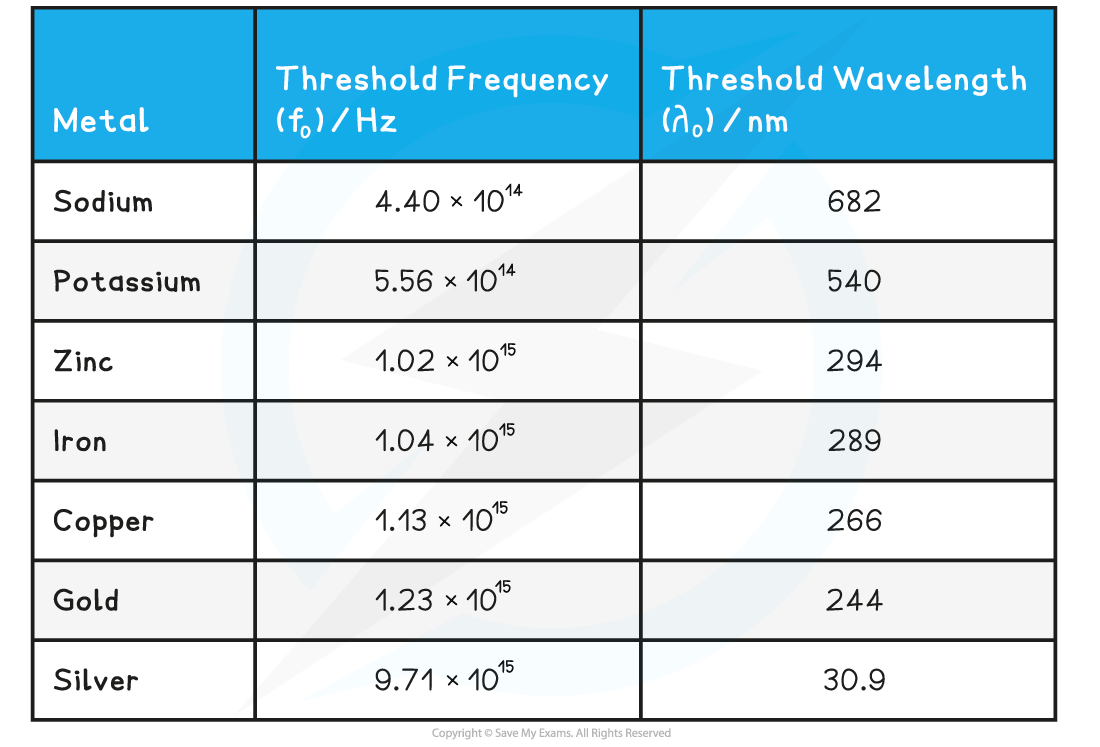
- Threshold frequency and wavelength are properties of a material, and vary from metal to metal
Threshold frequencies and wavelengths for different metals
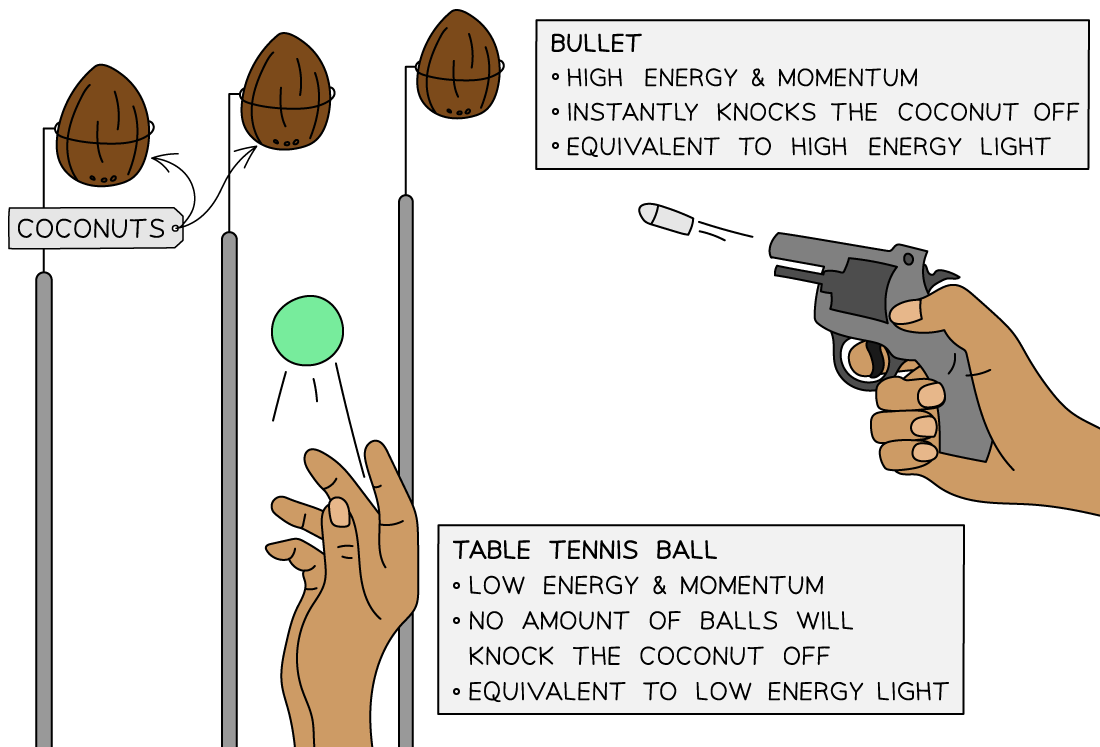
Exam Tip
A useful analogy for threshold frequency is a fairground coconut shy:
- One person is throwing table tennis balls at the coconuts, and another person has a pistol
- No matter how many of the table tennis balls are thrown at the coconut it will still stay firmly in place – this represents the low frequency quanta
- However, a single shot from the pistol will knock off the coconut immediately – this represents the high frequency quanta
The Work Function
- The work function Φ, or threshold energy, of a material, is defined as:
The minimum energy required to release a photoelectron from the surface of a metal
- Consider the electrons in a metal as trapped inside an ‘energy well’ where the energy between the surface and the top of the well is equal to the work function Φ
- A single electron absorbs one photon
- Therefore, an electron can only escape from the surface of the metal if it absorbs a photon which has an energy equal to Φ or higher
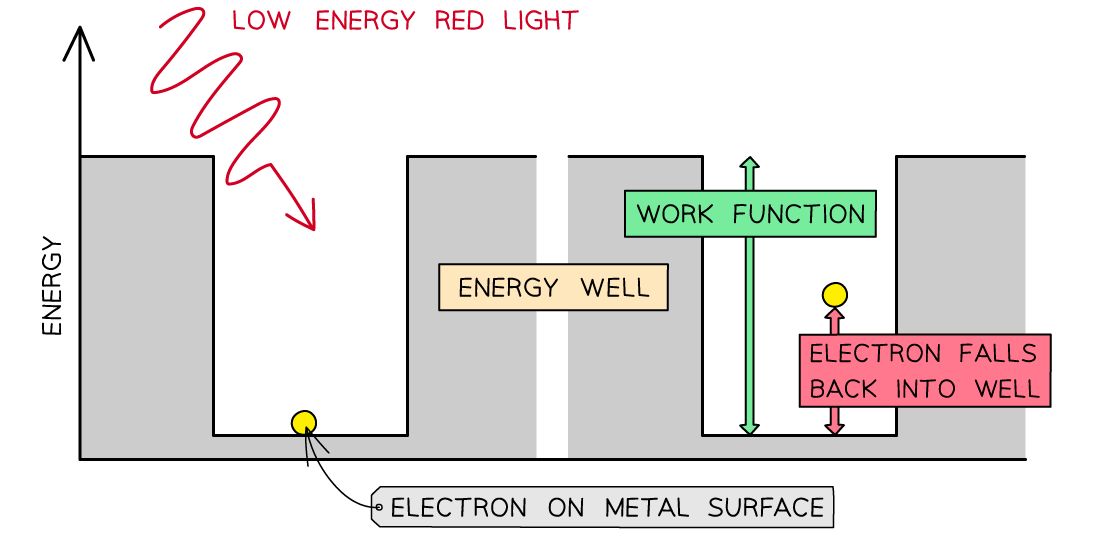
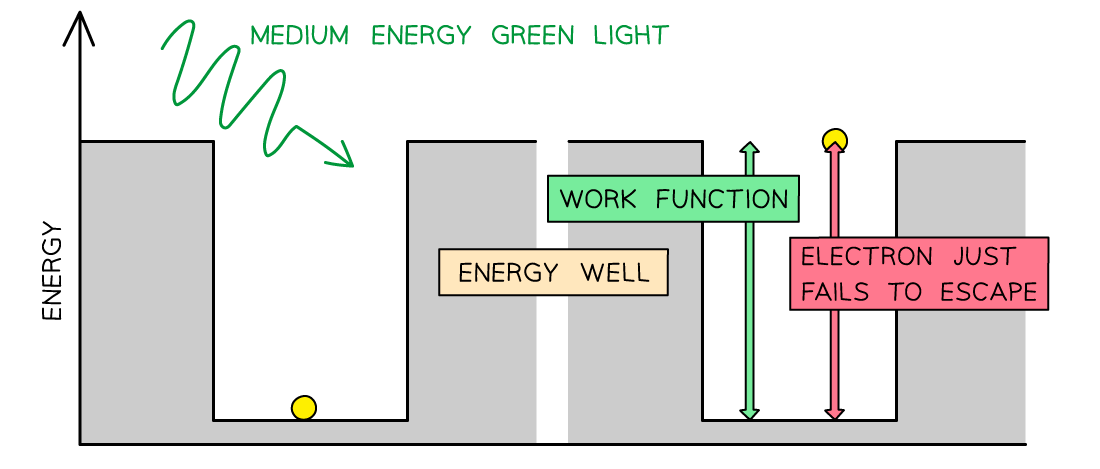
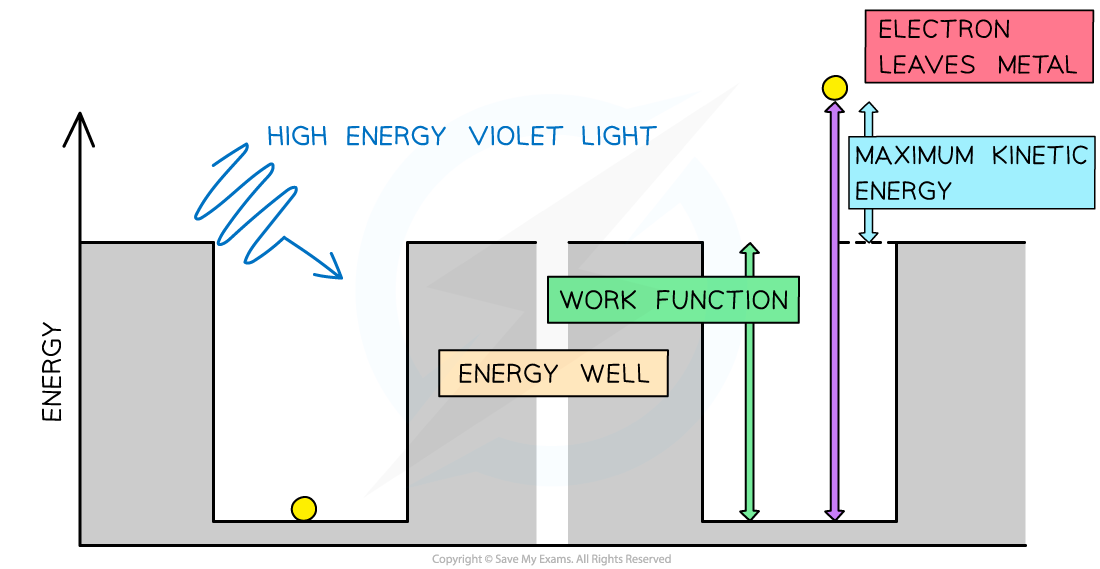
In the photoelectric effect, a single photon may cause a surface electron to be released if it has sufficient energy
- Different metals have different threshold frequencies and hence different work functions
- Using the well analogy:
- A more tightly bound electron requires more energy to reach the top of the well
- A less tightly bound electron requires less energy to reach the top of the well
- Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region
- This is because the attractive forces between the surface electrons and positive metal ions are relatively weak
- Transition metals, such as zinc and iron, have threshold frequencies in the ultraviolet region
- This is because the attractive forces between the surface electrons and positive metal ions are much stronger
Stopping Potential
- Stopping potential, Vs, is defined as:
The potential difference required to stop photoelectron emission from occurring
- The photons arriving at the metal plate cause photoelectrons to be emitted
- This is called the emitter plate
- The electrons that cross the gap are collected at the other metal plate
- This is called the collector plate
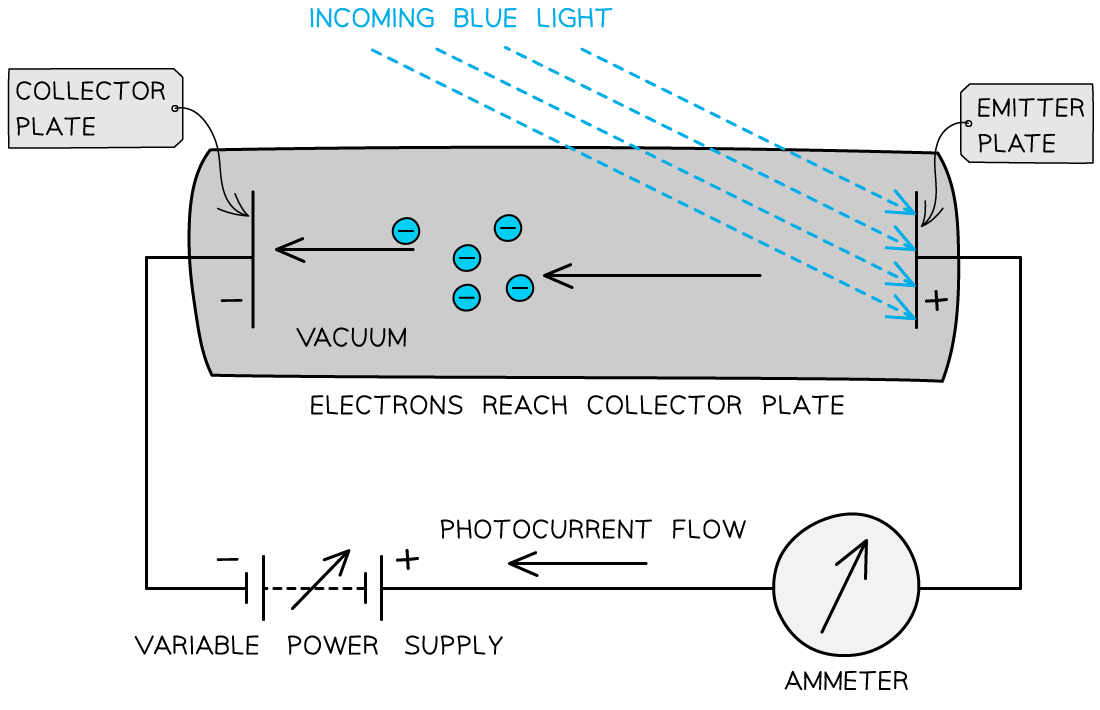
This set up can be used to determine the maximum kinetic energy of the emitted photoelectrons
- The flow of electrons across the gap results in an e.m.f. between the plates that causes a current to flow around the rest of the circuit
- Effectively, it becomes a photoelectric cell producing a photoelectric current
- If the e.m.f. of the variable power supply is initially zero, the circuit operates only on the photoelectric current
- As the supply is turned up, the emitter plate becomes more positive (because it is connected to the positive terminal of the supply)
- As a result, electrons leaving the emitter plate are attracted back towards it
- This is because the p.d. across the tube opposes the motion of the electrons between the plates
- If any electrons escape with enough kinetic energy, they can overcome this attraction and cross to the collector plate
- And if they don't have enough energy, they can't cross the gap
- By increasing the e.m.f. of the supply, eventually a p.d. will be reached at which no electrons are able to cross the gap – this is the stopping potential, Vs
- At this point, the energy needed to cross the gap is equal to the maximum kinetic energy KEmax of the electrons
KEmax = eVS
Exam Tip
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








