- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记2.1.4 Alpha & Beta Decay
α & β Decay Equations
Alpha Decay
- Alpha decay is common in large, unstable nuclei with too many protons
- The decay involves a nucleus emitting an alpha particle and decaying into a different nucleus
- An alpha particle consists of 2 protons and 2 neutrons (the nucleus of a Helium atom)
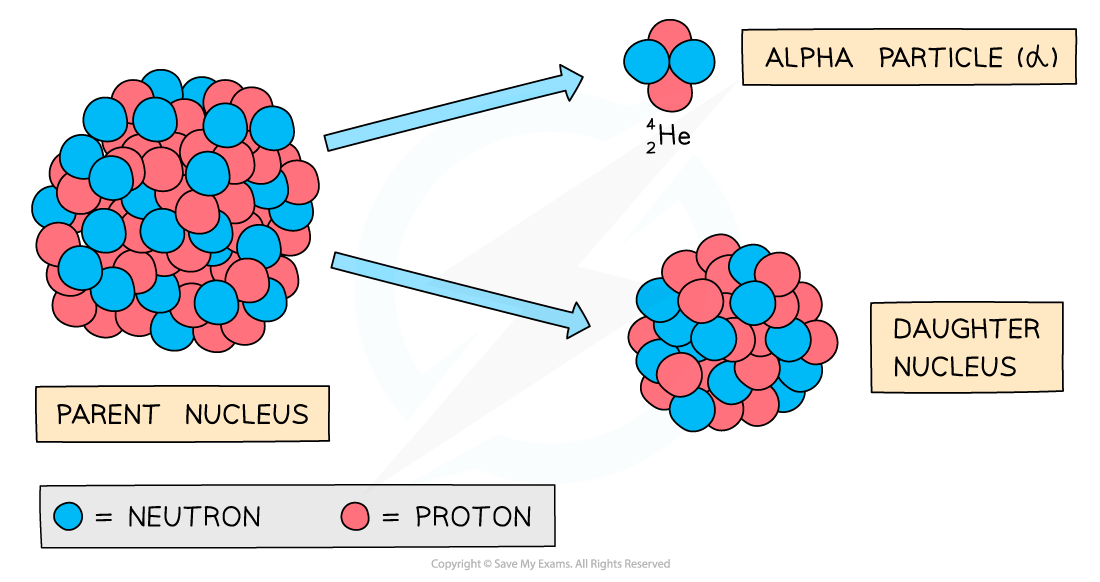
Alpha decay produces a daughter nucleus and an alpha particle (helium nucleus)
- When an unstable nucleus (the parent nucleus) emits radiation, the constitution of its nucleus changes
- As a result, the isotope will change into a different element (the daughter nucleus)
- Alpha decay can be represented by the following radioactive decay equation:

Alpha decay equation
- When an alpha particle is emitted from a nucleus:
- The nucleus loses 2 protons: proton number decreases by 2
- The nucleus loses 4 nucleons: nucleon number decreases by 4
Worked Example
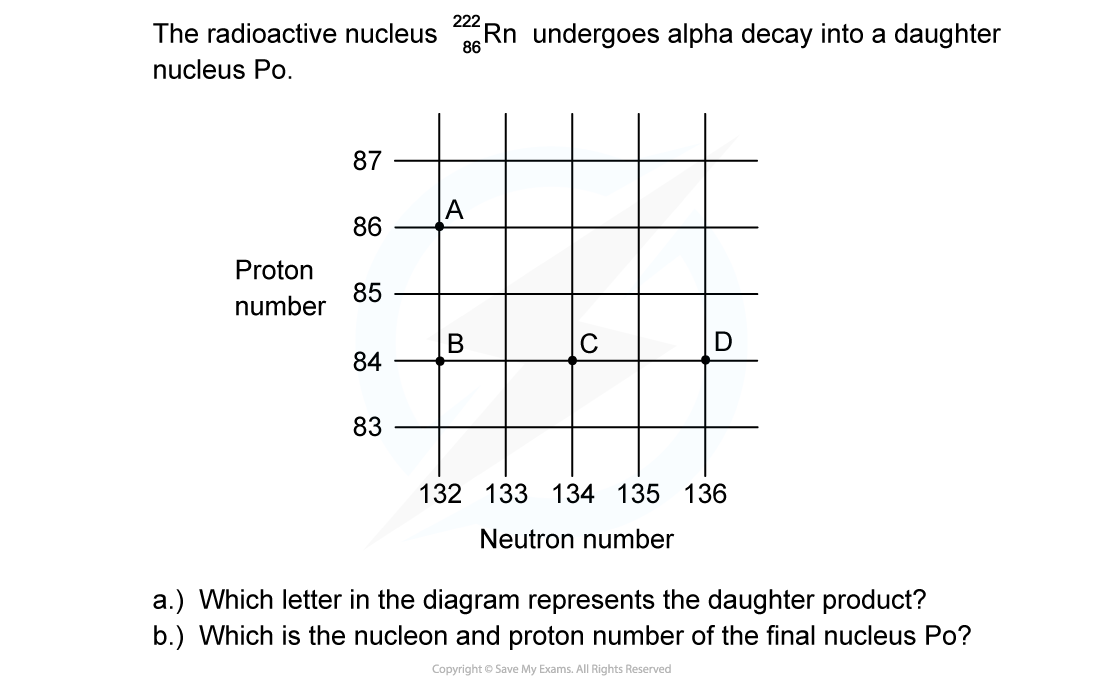
ANSWER: C
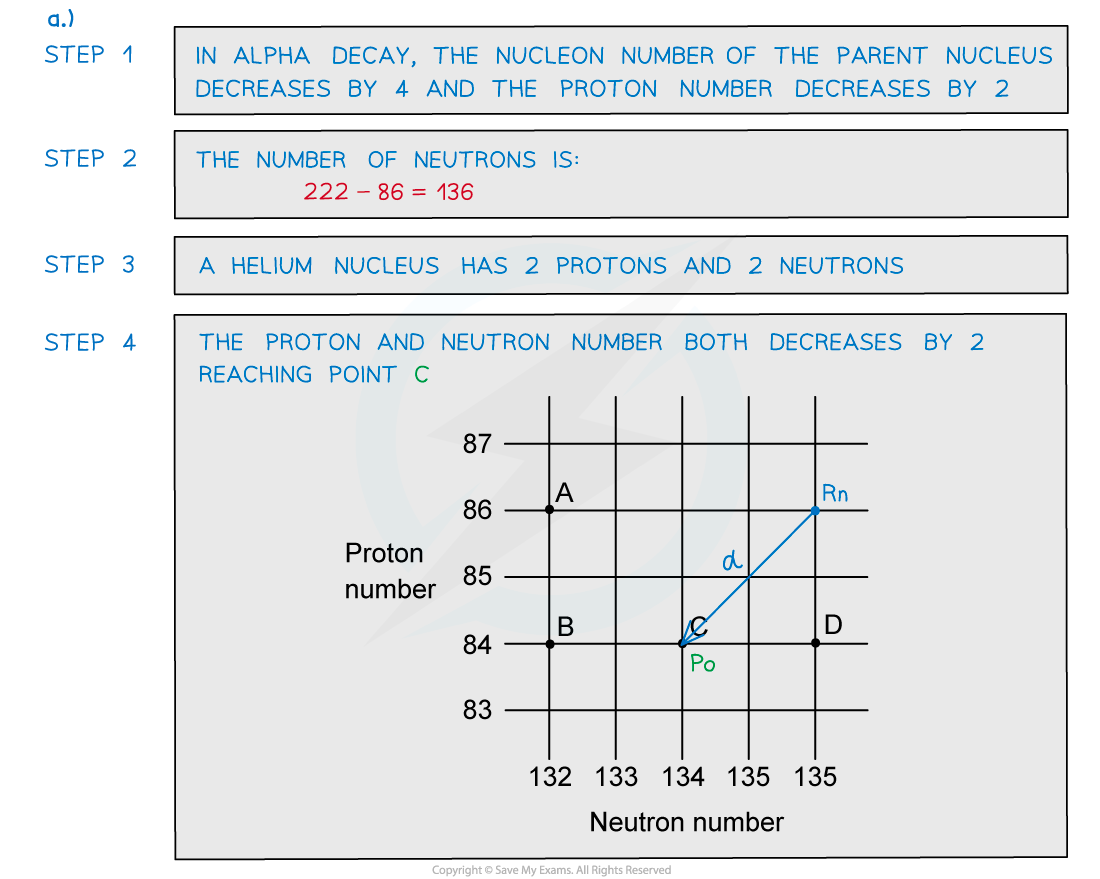
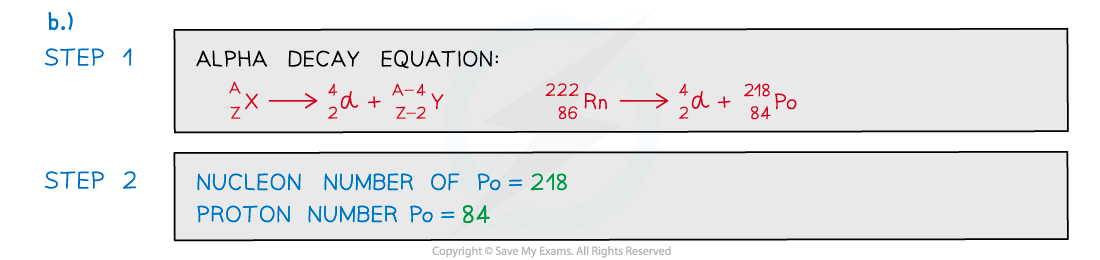
Beta-Minus Decay
- A beta-minus, β-, particle is a high energy electron emitted from the nucleus
- β- decay is when a neutron turns into a proton emitting an electron and an anti-electron neutrino
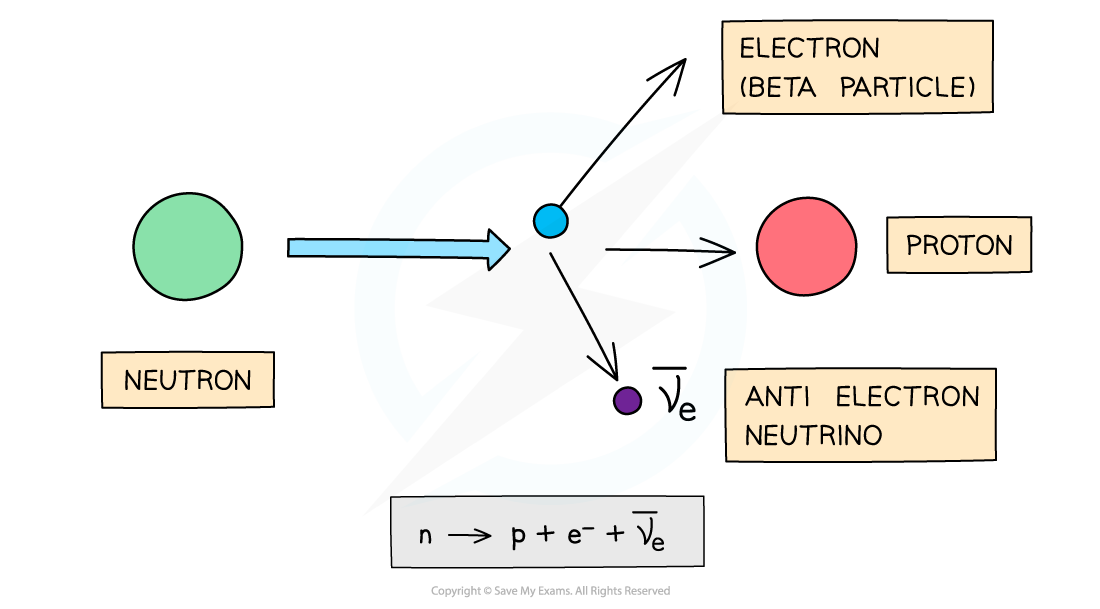
- When a β- particle is emitted from a nucleus:
- The number of protons increases by 1: proton number increases by 1
- The total number of nucleons stays the same: nucleon number remains the same

Equation for beta minus emission
- The new nucleus formed from the decay is called the “daughter” nucleus (nitrogen in the example above)
Beta-Plus Decay
- A beta-plus, β+, particle is a high energy positron emitted from the nucleus
- β+ decay is when a proton turns into a neutron emitting a positron (anti-electron) and an electron neutrino
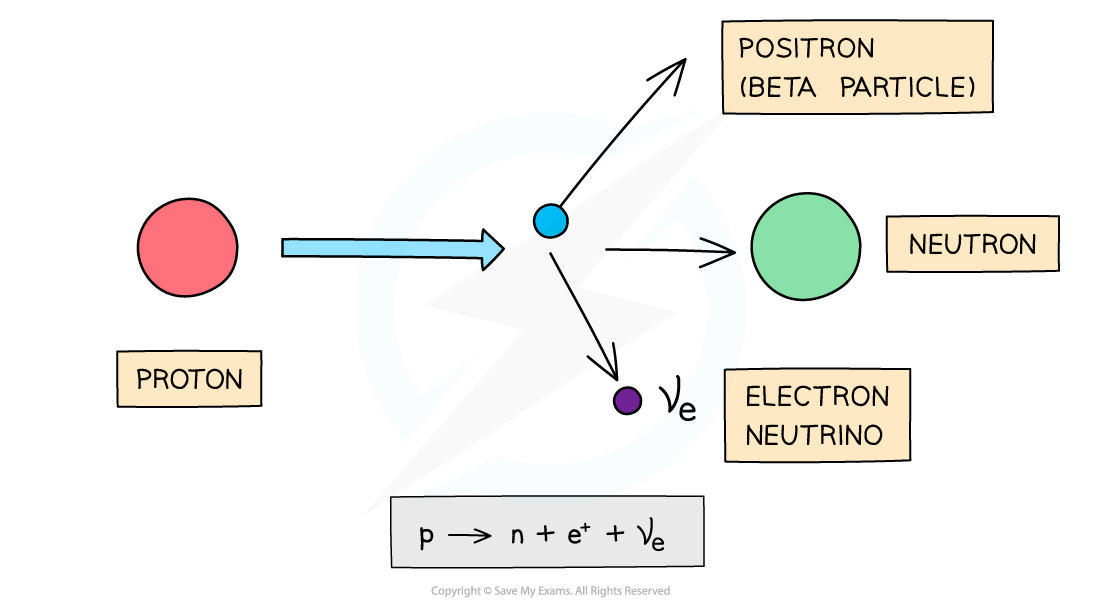
- When a β+ particle is emitted from a nucleus:
- The number of protons decreases by 1: proton number decreases by 1
- The total number of nucleons stays the same: nucleon number remains the same

Equation for beta plus emission
Worked Example
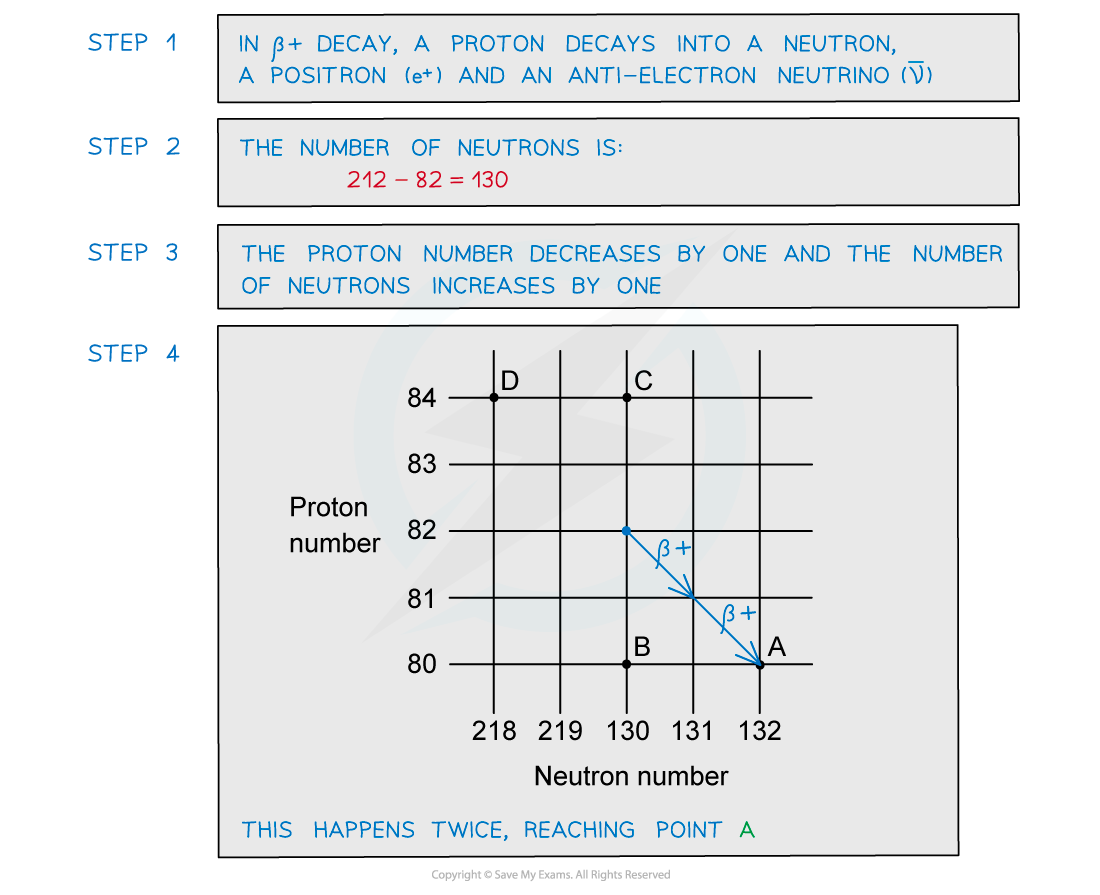
A radioactive substance with a nucleon number of 212 and a proton number of 82 decays by β-plus emission into a daughter product which in turn decays by further β-plus emission into a granddaughter product.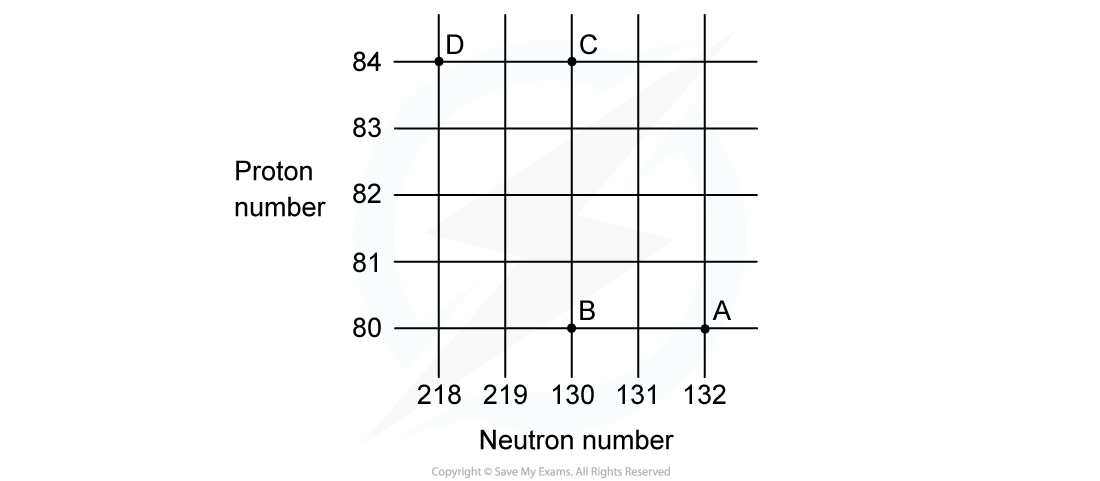 Which letter in the diagram represents the granddaughter product?
Which letter in the diagram represents the granddaughter product?
ANSWER: A
Exam Tip
Remember to avoid the common mistake of confusing the number of neutrons with the nucleon number. In alpha decay, the nucleon (protons and neutrons) number decreases by 4 but the number of neutrons only decreases by 2.
Neutrino Emission
- An electron neutrino is a type of subatomic particle with no charge and negligible mass which is also emitted from the nucleus
- The anti-neutrino is the antiparticle of a neutrino
- Electron anti-neutrinos are produced during β– decay
- Electron neutrinos are produced during β+ decay
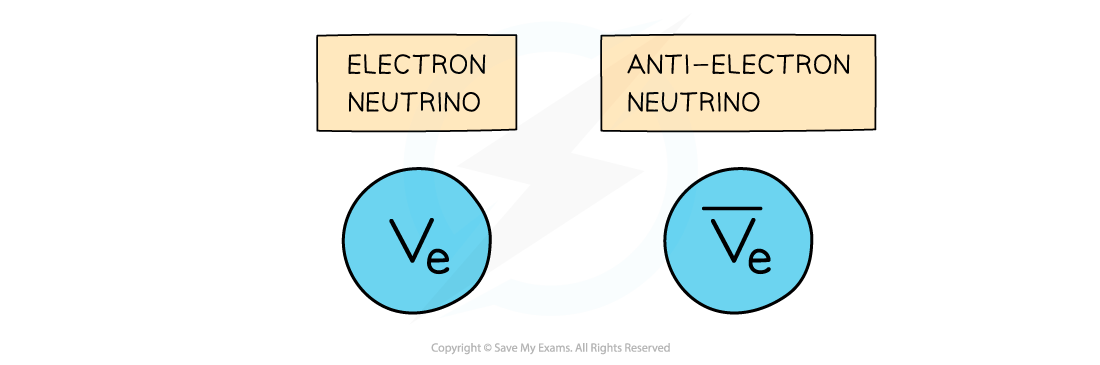
- Although the neutrino has no charge and negligible mass, its existence was hypothesised to account for the conservation of energy in beta decay
Exam Tip
One way to remember which particle decays into which depends on the type of beta emission, think of beta ‘plus’ as the ‘proton’ that turns into the neutron (plus an electron neutrino)
转载自savemyexams


最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








