- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Physics复习笔记2.1.2 Nucleon & Proton Number
AZX Notation
- A nuclide is a group of atoms containing the same number of protons and neutrons
- For example, 5 atoms of oxygen are all the same nuclide but are 5 separate atoms
- Atomic symbols are written in a specific notation called nuclide or AZX notation

Atomic symbols in AZX Notation describe the constituents of nuclei
- The top number A represents the nucleon number or the mass number
- Nucleon number (A) = total number of protons and neutrons in the nucleus
- The lower number Z represents the proton or atomic number
- Proton number (Z) = total number of protons in the nucleus
- Note: In Chemistry, the nucleon number is referred to as the mass number and the proton number as the atomic number. The periodic table is ordered by atomic number
Isotopes
- Although all atoms of the same element always have the same number of protons (and hence electrons), the number of neutrons can vary
- An isotope is defined as:
An atom (of the same element) that has an equal number of protons but a different number of neutrons
- Hydrogen has two isotopes: deuterium and tritium
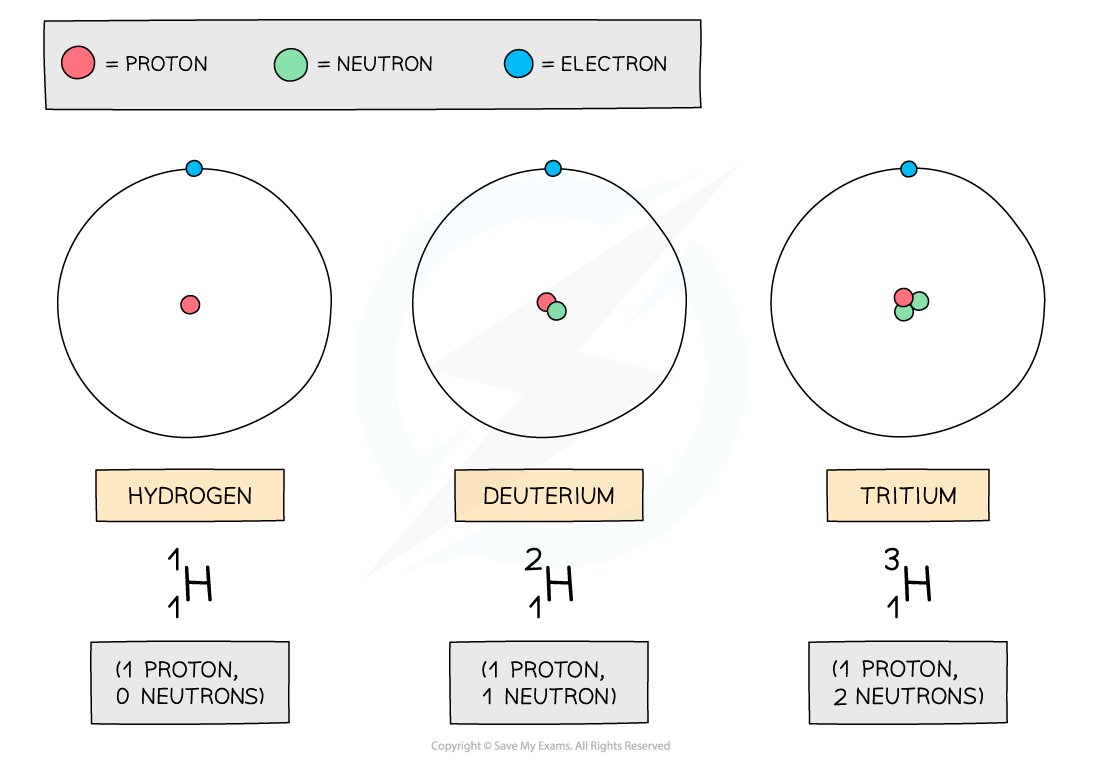
The three atoms shown above are all forms of hydrogen, but they each have different numbers of neutrons
- The neutron number of an atom is found by subtracting the proton number from the nucleon number
- Since nucleon number includes the number of neutrons, an isotope of an element will also have a different nucleon / mass number
- Since isotopes have an imbalance of neutrons and protons, they are unstable
- This means they constantly decay and emit radiation to achieve a more stable form
- This can happen from anywhere between a few nanoseconds to 100,000 years
Isotopic Data
- Isotopic data is defined as:
The relative amounts of different isotopes of an element found within a substance
- It is used to identify an isotopic signature within organic and inorganic materials
- Isotopic data is often used for determining the age of archaeological findings and is used in radioactive dating
- Carbon–14 is a naturally occurring isotope most often used for this, since it is present in all living beings and undergoes radioactive decay
- When a plant or animal dies, the natural decay of this isotope means the concentration of the carbon–14 in its tissue gradually reduces
- Since carbon–14 has a long half-life of around 6000 years, the half-life can be used to determine the age of the plant or animal when it died
Worked Example
One of the rows in the table shows a pair of nuclei that are isotopes of one another.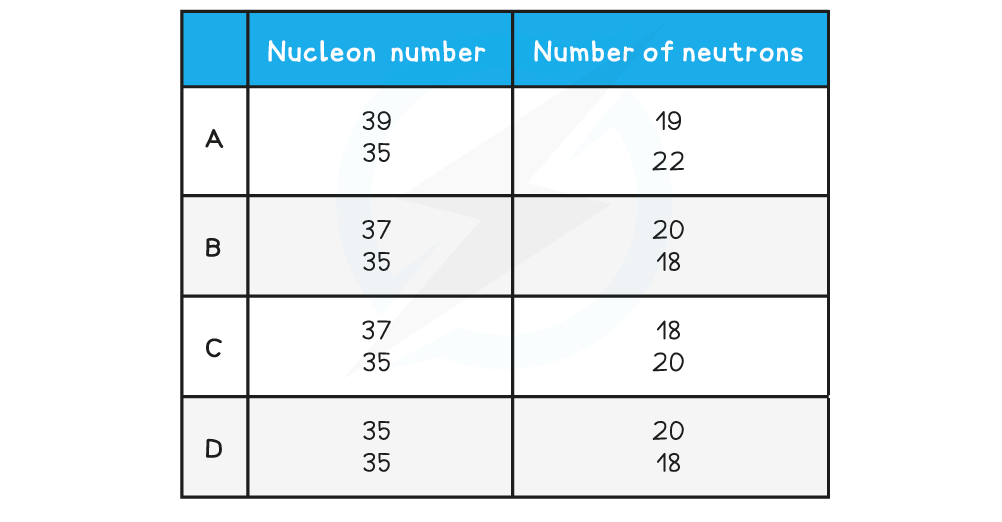 Which row is correct?
Which row is correct?
ANSWER: B
Step 1: Properties of isotopes
Isotopes are nuclei with the same number of protons but different number of neutrons
The nucleon number is the sum of the protons and neutron
Therefore, an isotope has a different nucleon number too
Step 2: Calculate protons in the first nucleus
Nucleon number: 37
Neutrons: 20
Protons = 37 − 20 = 17
Step 3: Calculate protons in the second nucleus
Nucleon number: 35
Neutrons: 18
Protons = 35 − 18 = 17
Step 4: Conclusion
Therefore, they have the same number of protons but different numbers of neutrons and are isotopes of each other
The correct answer is therefore option B
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1









