- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.3.2 Required Practical 10b
Preparation of an Ester
- This required practical covers the following mandatory skills
- use water bath or electric heater or sand bath for heating
- use laboratory apparatus for a variety of experimental techniques
- purifying a liquid, including the use of a separating funnel
- safely and carefully handle solids and liquids
- The preparation of ethyl ethanoate is a suitable experiment that gives opportunities to perform these skills
Key steps in the procedure
- A mixture of 10 cm3 ethanol, 12 cm3 glacial ethanoic acid and 15 drops of concentrated sulfuric acid are added to a pear shaped flask
- A few anti-bumping granules are added to the flask and the flask is partially immersed in a beaker of water
- A condenser is added in the vertical position and clamped in place, so that it is set up for heating under reflux
- The water is heated and the reaction mixture allowed to boil for about 15 minutes
- The heated is stopped and the mixture allowed to cool back to room temperature
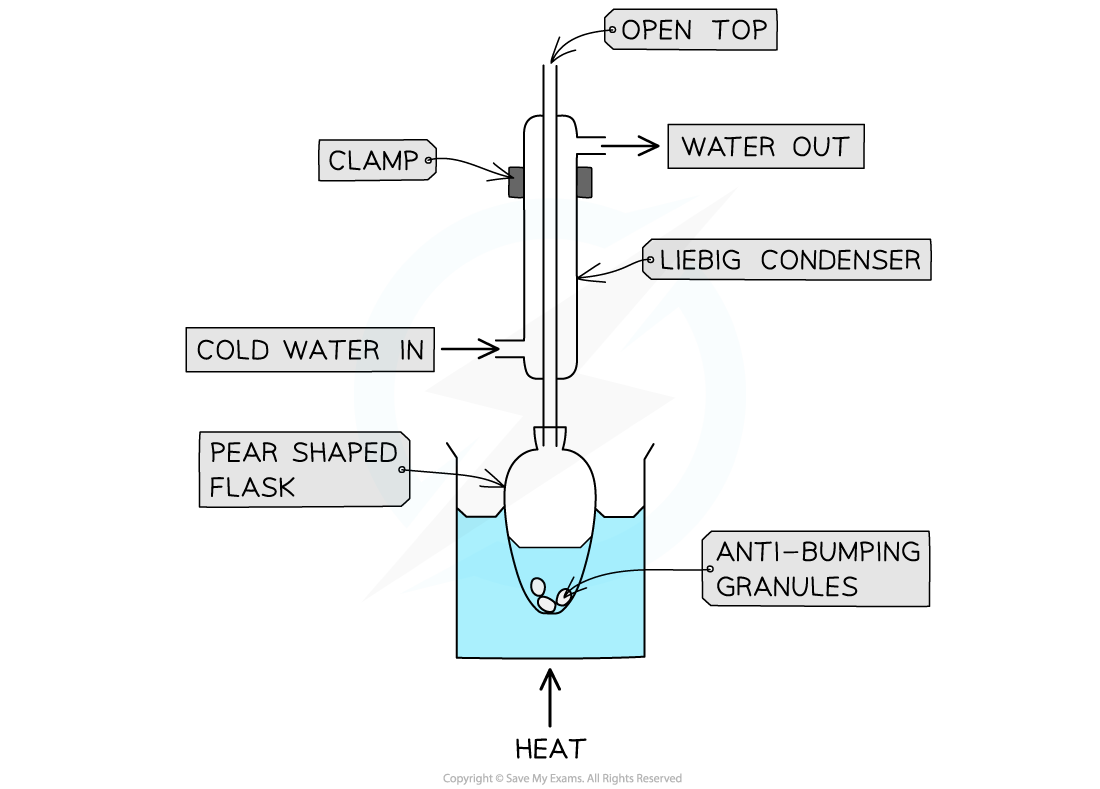
The preparation of ethyl ethanoate involves heating under reflux for about 15 minutes
Separation of the product
- The reaction mixture is poured into a beaker containing saturated sodium carbonate solution
- This neutralises the unreacted acids and helps to separate the ester into an oily layer
- The contents of the beaker are transferred to a separating funnel and a stopper added
- The separating funnel is inverted and the stopcock opened to release the pressure - this is repeated 15-20 times
- The two layers are allowed to separate and the ethyl ethanoate floats on top of the aqueous solution
- The stop cock is opened so that the aqueous layer drains away and then the ethyl ethanoate can be drained into a clean beaker
- A little solid sodium sulfate is added to absorb water and dry the ethyl ethanoate
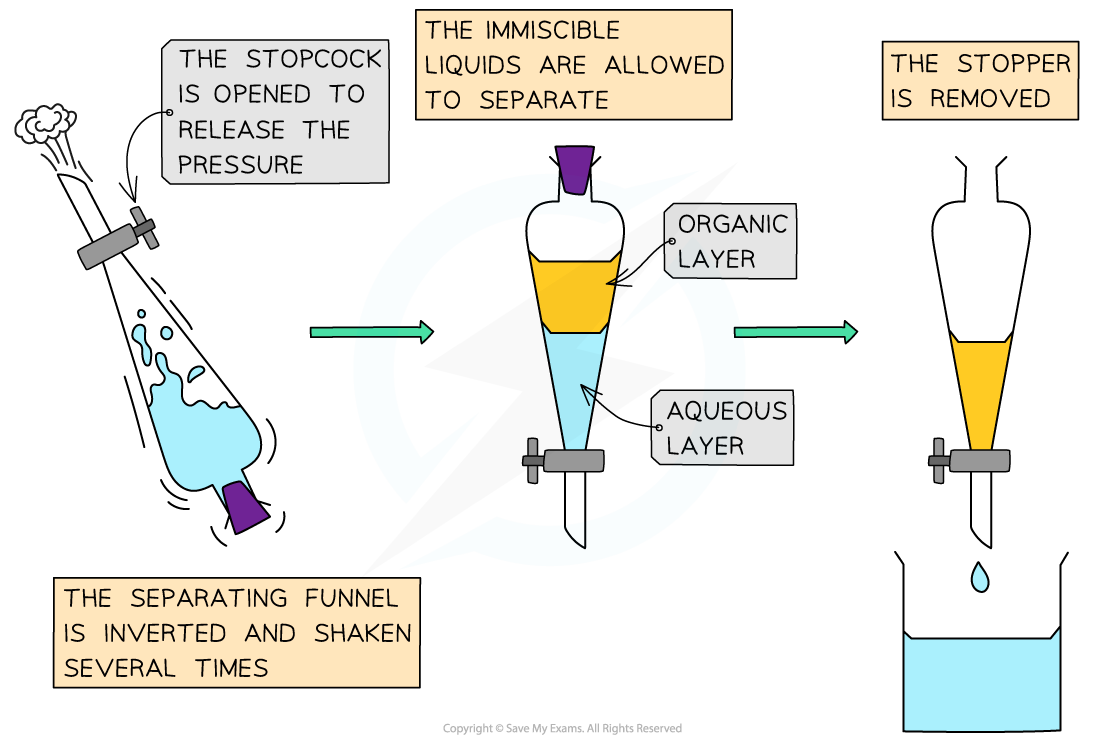
A separating funnel allows to the product to be cleaned and isolated
Purification of the product
- The ethyl ethanoate is purified by distillation
- The ester has lost the hydrogen bonds that were present in the alcohol and carboxylic acid, so it has a lower boiling point than the two reactants
- The distillate is collected and the temperature at which it comes off is noted
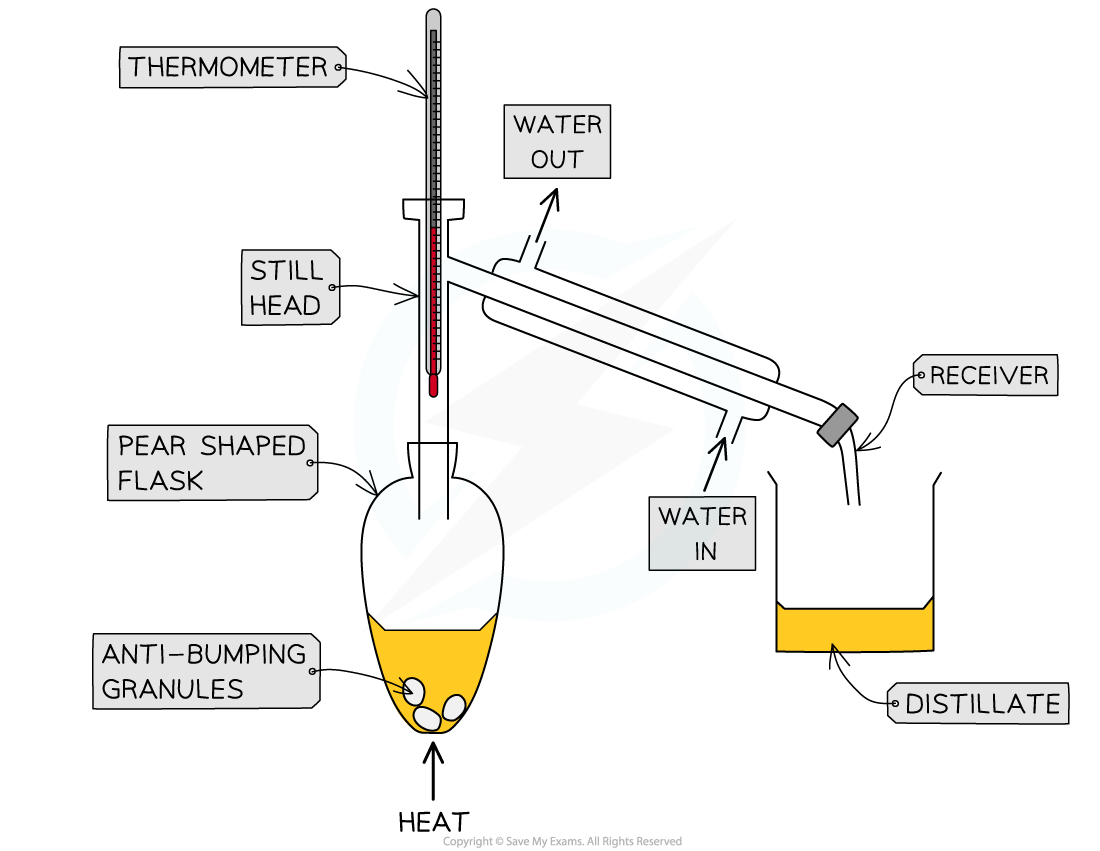
The ester is purified by distillation
Key Hazards
- The concentrated sulfuric and ethanoic acids are very corrosive so safety glasses and gloves should be worn
- Ethanol is flammable so care should be taken with naked flames
- Ethyl ethanoate is toxic and flammable and has a strong odour so should be handled in a fume cupboard
Identifying an Ester
- The boiling point of an ester can be used as one piece of information to establishing its identity
- The ester can then be hydrolysed and the resulting carboxylic acid purified by recrystallisation and its melting pint checked against known values
- The acid hydrolysis of an ester is very slow, so it must be heated under reflux with an acid catalyst to make the carboxylic acid
- Methyl benzoate makes a suitable choice of ester for hydrolysis and identification
- The hydrolysis reaction is
C6H5COOCH3 + H2O → C6H5COOH + CH3OH
Key steps in the procedure
- 2.0g methyl benzoate is added to a pear-shaped flask along with 40 cm3 of 1.0 mol dm-3 sulfuric acid
- The reaction mixture is heated under reflux for 30 minutes
- While still warm, the mixture is poured into a beaker of cold water which causes the benzoic acid to precipitate out
- The solid is recovered by Buchner filtration and recrystallised using hot water
- The melting point of the solid is measured and matched up to a data book value
- Expected melting point benzoic acid = 122 oC
- Expected boiling point of methyl benzoate = 199 oC
Exam Tip
Esters have characteristic pleasant odours, but smell alone is not sufficient to identify them, so boiling point of the ester or melting point of the derived carboxylic acid can be used.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








