- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.2.9 Reactions of Metal-Aqua Ions
Reactions of Metal-Aqua Ions
- In this practical activity, test tube reactions of copper(II), iron(II), iron(III) and aluminium ions with three bases are investigated
- 10 drops of the metal-aqua ion are added to a clean dry test tube
- 10 drops of 1.0 mol dm-3 sodium hydroxide solution are added, the test tube shaken and any observations noted
- A further 10 drops of the base are added so that the base is in excess and any additional observations are recorded
- The procedure is repeated with 1.0 mol dm-3 ammonia solution and then 1.0 mol dm-3 sodium carbonate solution
The reactions of metal-aqua ions with bases table
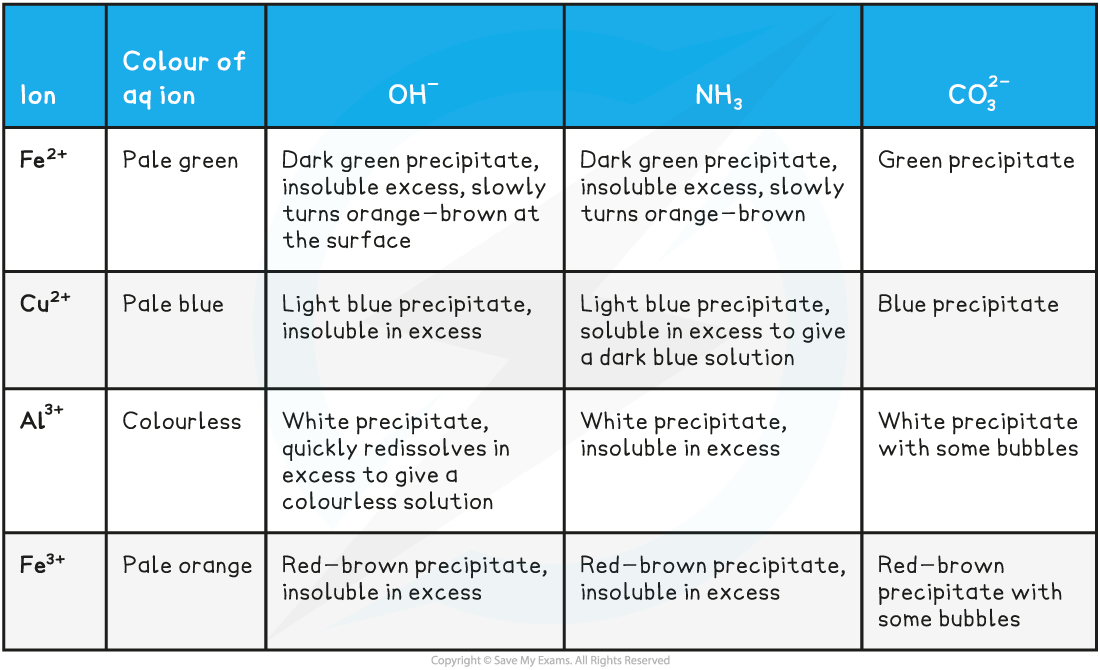
- It is important to use the correct vocabulary in your observations when referring to solutions and precipitates as well as mentioning further changes on addition of an excess reagent
- Some changes take place over the course of a few minutes so its best not to dispose of the contents of test tubes right away, but return to them for another look
- The identities of the precipitates and the equations for the reactions are covered in a previous section on metal-aqua ions
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








