- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.2.5 Vanadium Oxidation States
Vanadium Oxidation States
Vanadium Oxidation States
- Vanadium is a transition metal which has variable oxidation states
- Vanadate(V) ions can be reduced to oxidised vanadium species with the oxidation states II, III and IV by using zinc in acidic conditions
- You could be asked to carry out a test tube practical to observe the different colour changes and calculate the oxidation state of the vanadium species produced
Practical
- Add a quarter of a spatula of ammonium vanadate(V) to a test tube
- Using a dropping pipette, half-fill the test tube with 1.0 mol dm−3 hydrochloric acid and shake gently
- Note your observations
- Add one small piece of zinc, and gently shake the test tube
- Note your observations over a period of 15 minutes
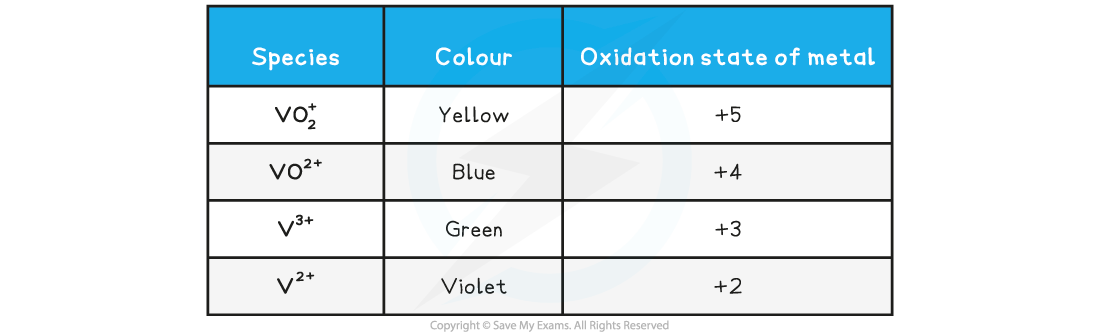
Vanadium results table
Exam Tip
V2+ is oxidised in air, therefore to observe the V2+, the test tube may need a stopper once the reduction has begun. Otherwise it turns back green because of its contact with oxygen in the air. It is oxidised back to vanadium(III).
Worked Example
What do you observe as ammonium vanadate(V) is reduced?
Answer:
The solid dissolves in the acid to form a yellow solution. This becomes a blue, then green and finally violet solution
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









