- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.2.3 Ligand Substitution Experiments
Ligand Substitution Experiments
Ligand Substitution Experiments
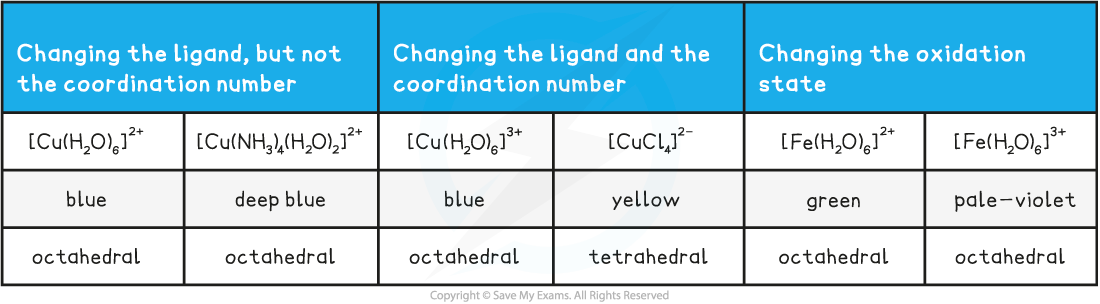
- The factors that affect the colour formed for different transition metal ion solutions are:
- type of ligand
- change in oxidation state
- co-ordination number
- These colour changes can be observed by performing test tube reactions of metal aqua ions in test tubes with addition of different reagents
Exam Tip
It is important with test tube reactions to not only record the colour in your observations but also whether a solution or precipitate is formed.
Test tube reactions
- Measure 2 cm3 of 1.0 mol dm−3 aqueous metal aqua ion solution and transfer into a test tube, held in the test tube rack
- Using a dropping pipette, add the 1.0 mol dm −3 ammonia solution dropwise, and gently shake the test tube between drops and record any observations
- Continue to add the 1.0 mol dm−3 ammonia solution and shake between additions and record any observations
- This procedure can be repeated with different aqueous metal aqua ion solutions and reagents e.g. hydrochloric acid
Table showing factors affecting colour
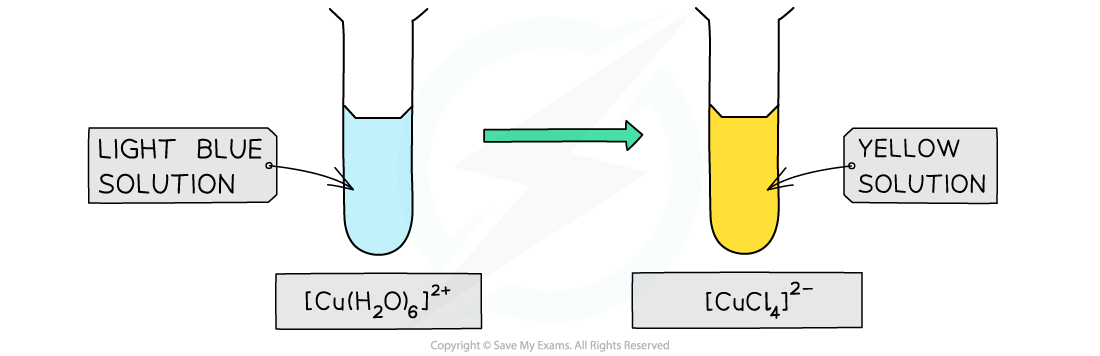
The colour changes from light blue to a yellow-green when copper(II) is treated with concentrated hydrochloric acid. The green appearance is due to the presence of unreacted aqueous copper(II) ions
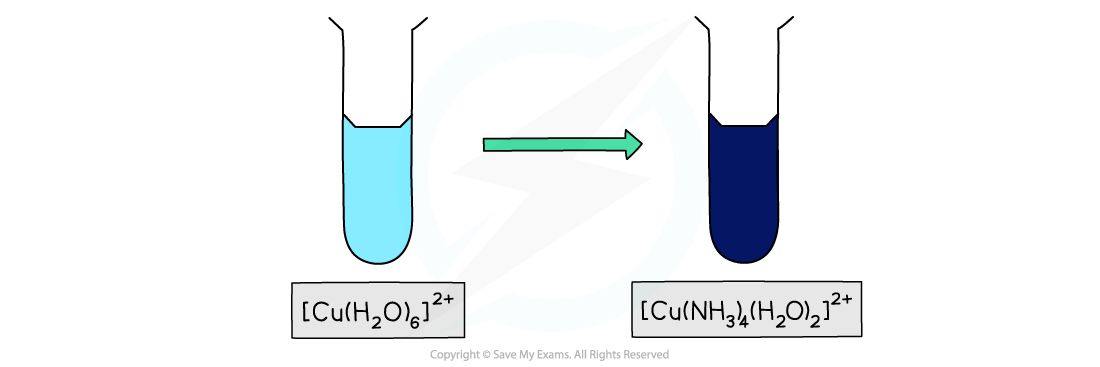
Addition of excess aqueous ammonia to the aqueous copper(II) ion results in a gorgeous deep blue complex
Bidentate and multidentate
Bidentate and multidentate
- Complexes involving bidentate and even more so, multidentate ligands are more stable than those with only monodentate ligands in them
- The underlying reason for this is that each multidentate ligand displaces more than one water molecule
- This leads to an increase in the number of species present in the system, and therefore an increase in entropy
- An increase in entropy makes the formation of the chelated complex more favourable
- This is known as the chelate effect
Rate of substitution
- UV/visible spectrophotometry could be used to compare the rate of ligand substitution of a metal aqua ion of monodentate and multidentate ligands
- The Lambert-Beer law relates the absorbance to the concentration of coloured complex ions in solution
- You don't need to recall this law for the exam but it can be used along with UV/visible spectrophotometer to show the mathematics behind working out the rate of substitution
- A = ɛcl
- A = absorbance, c = concentration, ɛ = molar extinction coefficient and l = path length (distance travelled by radiation through the solution)
- You don't need to recall this law for the exam but it can be used along with UV/visible spectrophotometer to show the mathematics behind working out the rate of substitution
Practical procedure
- The initial concentration of the metal aqua ion solution is measured using the UV/visible spectrophotometer, often this can be a simpler colorimeter
- An aqueous solution of monodentate ligand is added to the metal aqua ion solution
- Every 30 seconds, 0.1 cm3 reaction mixture is extracted and diluted with 100 cm3 of water to stop the reaction
- The sample is placed into a cuvette and radiation at the wavelength of maximum absorbance is passed through the sample
- The absorbance is recorded
- The process is repeated every 30 seconds for 300 seconds
- A calibration curve of time against absorbance can then be plotted
- The process can be repeated with the same metal aqua ion solution with a multidentate ligand
- The molar extinction coefficient, ɛ, can be calculated and used to compare the rate of ligand substitution of monodentate to multidentate ligand
Exam Tip
Sometimes the solution is too dilute to give a sufficiently intense colour for the colorimeter to measure. In such cases a suitable ligand is first added (e.g. thiocyanate ions, SCN-) to give an intense colour.The visible range is scanned to find the most intense absorption frequency to pass through the solution of a fixed path length.
转载自savemyexams

最新发布
© 2026. All Rights Reserved. 沪ICP备2023009024号-1