- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.2.2 Testing Period 3 Oxides
Testing Period 3 Oxides
Forming Period 3 Oxides
- Period 3 elements from sodium to sulfur react with oxygen, forming an oxide with the period 3 element in its highest possible oxidation state
- The metallic elements Na, Mg and Al are all highly reactive and when heated with oxygen they glow brightly
- These oxides form ionic lattices with sodium and magnesium oxides being basic and aluminium oxide is amphoteric (can acts as both an acid and base)
- Non metallic elements silicon, phosphorus and sulfur all react with oxygen forming molecular compounds with covalent bonding
- White phosphorus spontaneously catches fire and burns in air (red phosphorus also reacts but less vigorously)
- Sulfur initially forms SO2 and further oxidation to SO3 is slow unless a vanadium catalyst is used, so this period 3 element does not always use its highest possible oxidation state, unlike the other elements
Testing Period 3 Oxides
- As part of this topic you could be asked to perform/observe these reactions and you will need to know the following:
- Observations when reacted with oxygen
- Equations
- Typical pH of the resulting aqueous solutions of oxides
- Reactions of period 3 elements can be performed using a combustion jar and deflagrating spoon to lower the elements into oxygen (Na, Mg, S) or can be oxidised using tongs and a Bunsen burner for Al in the laboratory
- The resulting oxides can then be added to water and the pH tested using pH probe, pH paper or universal indicator
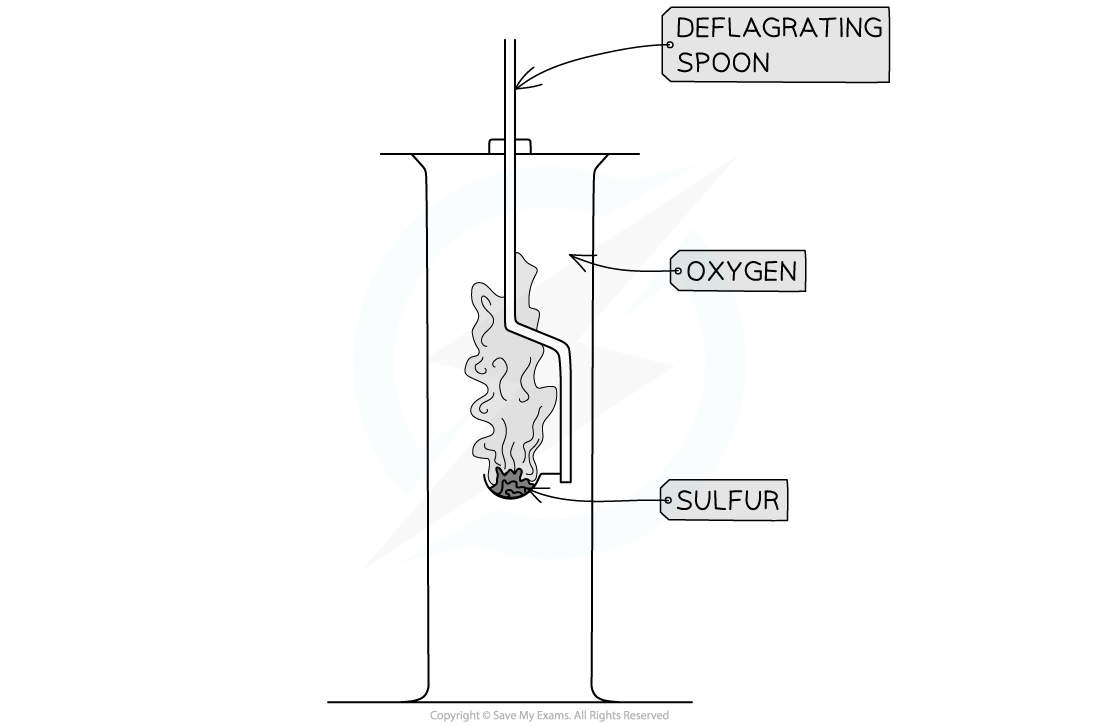
Combustion jar and deflagrating spoon used to lower sulfur into a jar containing oxygen gas
Table showing the properties of oxides in Period 3
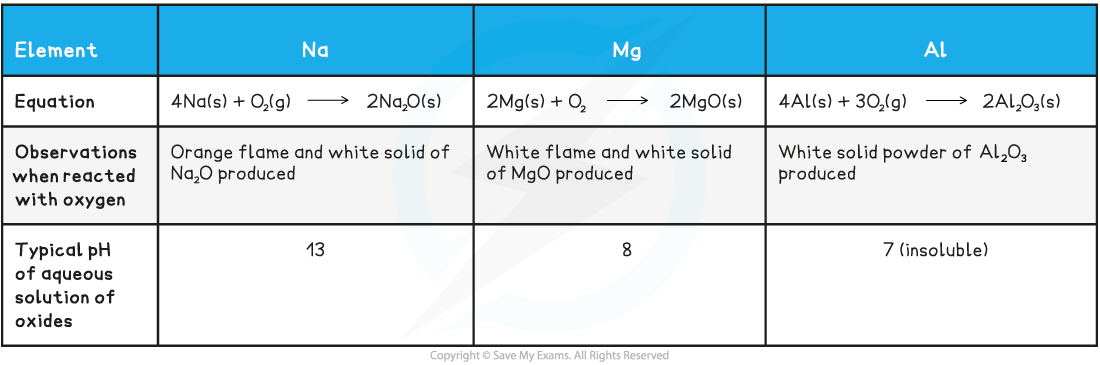
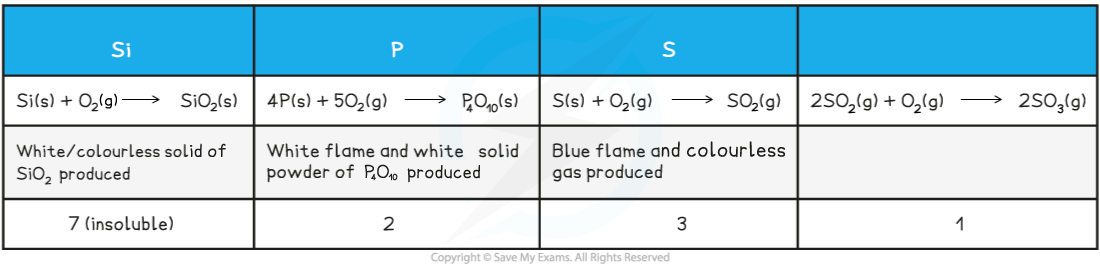
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








