- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.1.7 Making Simple Cells
Making Simple Cells
- Simple cells can be used to investigate the effect of different electrode combinations on the EMF produced by an electrochemical cell
Key steps in the procedure
- The metal foil electrodes are cleaned using sandpaper and rinsed under a cold running tap
- The metal foil is immersed into a solution of its ions and can be secured in place by folding the end over the lip of the beaker
- A strip of filter paper is soaked in saturated potassium chloride or nitrate solution to form the salt bridge; the ends of the strip are then immersed in metal ion solutions
- The EMF of the cells can then be measured and compared to the expected voltage from standard electrode potentials
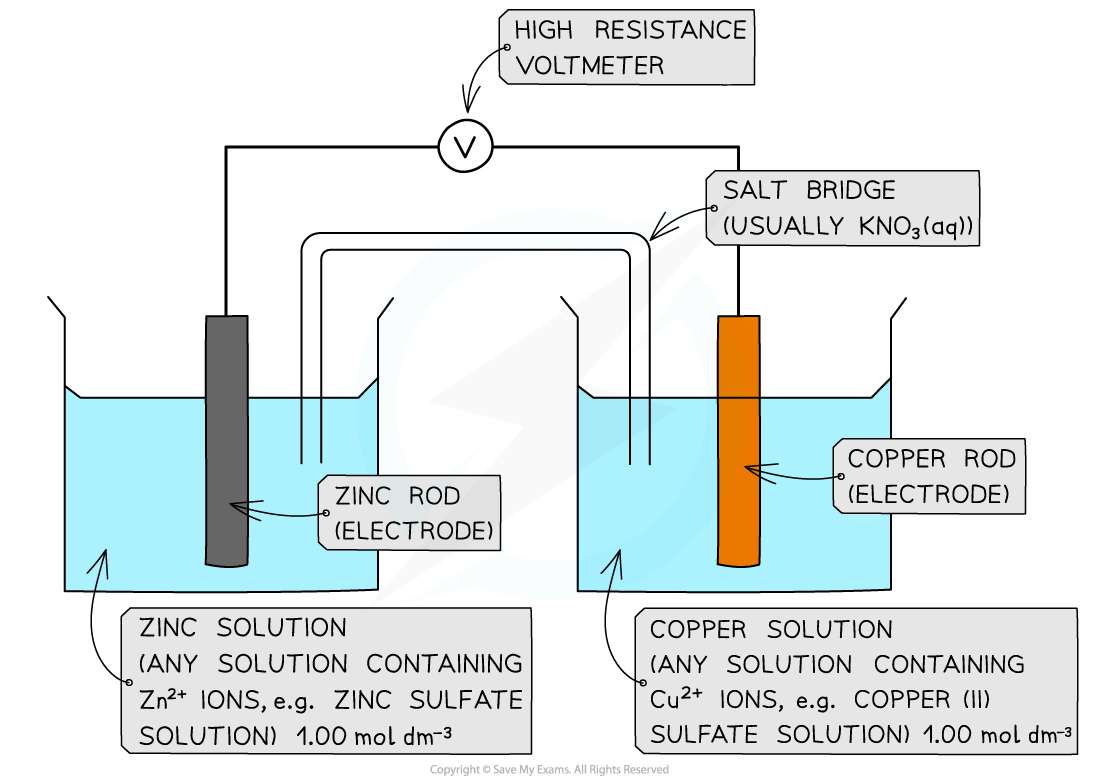
Measuring the EMF of different metal electrode combinations
Measured EMF specimen results table
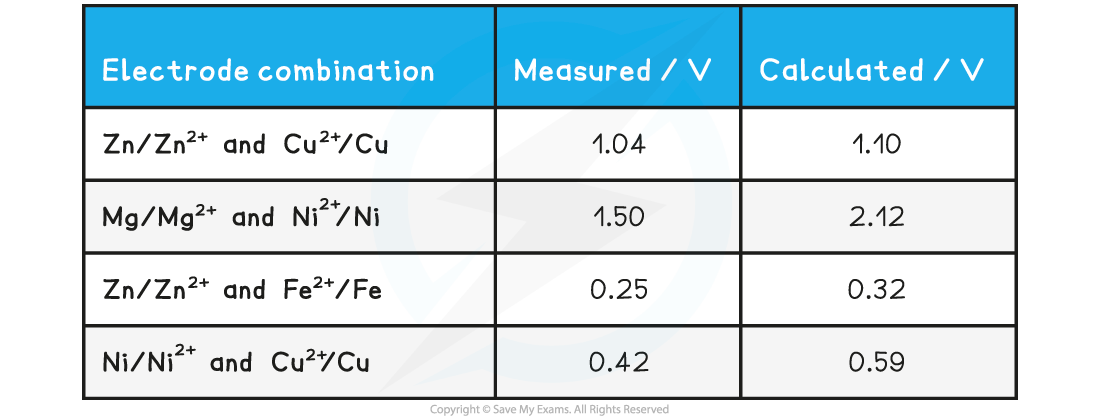
- Measured results in the lab are generally lower than calculated results from data tables
- This is usually because non-standard conditions have been used
Exam Tip
An electrode always contains a metal in contact with a solution of its own ions.By convention, EMF values are positive and the more negative electrode is written on the left.When calculating the EMF, subtract the right-hand electrode potential from the left hand one.Electrode equations are always written as reduction processes
Changing Conditions in Simple Cells
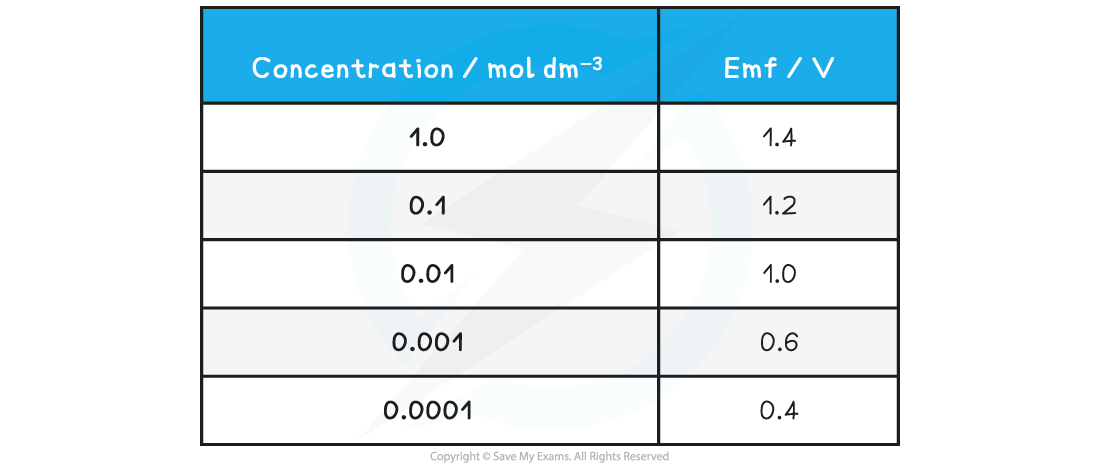
- The same procedure as the previous experiment can be used to investigate the effect of concentration on the EMF of an electrochemical cell
- At the end of the first measurement one of the solutions is diluted by a factor of ten by taking 25 cm3 of the solution and diluting it to 250 cm3 with distilled water using a large measuring cylinder
- The EMF is measured and the process is repeated until a total of five dilutions have been carried out
Practical tip
- Although in principle any pair of metals be used its best to use metals with a sufficiently large difference in electrode potential for this experiment so the range of results in meaningful
Concentration and EMF specimen results table
Analysis
- A graph can be plotted of EMF versus dilution factor:
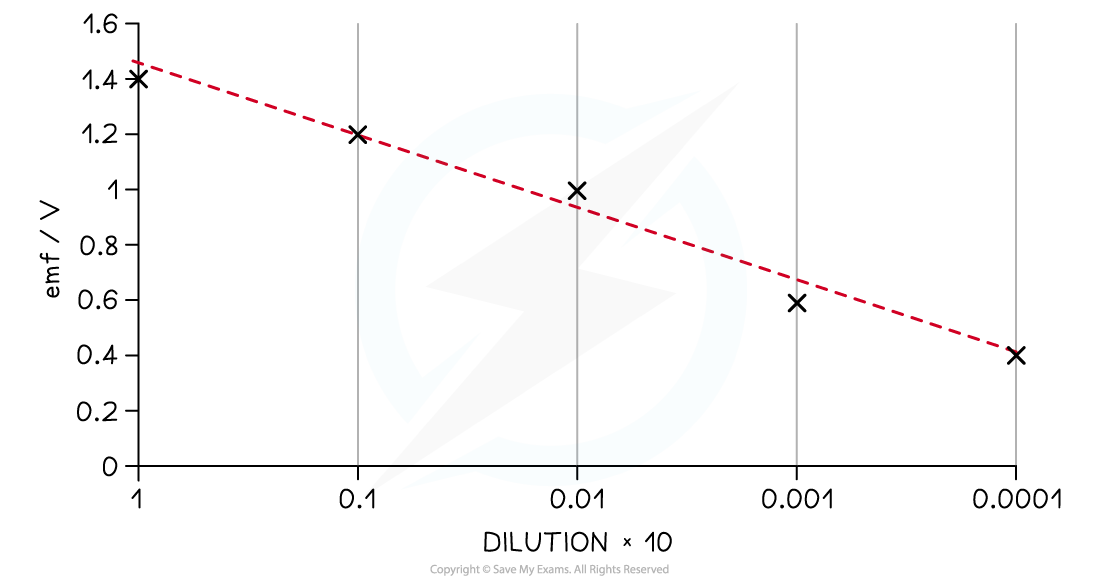
Graph of EMF against dilution for an electrochemical cell
- The graph shows that the cell EMF is proportional to the dilution to the power of ten
- Decreasing the concentration of the solution reduces the cell EMF because there are fewer particles to react
Further investigations
- The same equipment can be used to investigate the effect on cell EMF of changing the temperature, the spacing between the electrodes and even the depth of the electrodes
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








