- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.1.6 Determination of Kc
Determination of Kc
- The determination of an equilibrium constant can be carried out in a school laboratory using an esterification reaction between ethanol and ethanoic acid in the presence of an acid catalyst
- The equation for the reaction is:
C2H5OH (l) + CH3COOH (l) ⇌ CH3COOCH2CH3 (l) + H2O (l)
- The composition of the equilibrium is determined by titrating against standard sodium hydroxide solution and deducing the number of moles of acid present at equilibrium
- Once the acid is known, the equilibrium concentrations of the other substances can be deduced and Kc determined
- A number of reaction flasks are made up and analysed so that an average value for Kc can be found
Key steps in the procedure
- 6.0 g of concentrated ethanoic acid and 6.2 g of ethanol are added to a conical flask- this is equivalent to 0.1 mol
- A drop of concentrated sulfuric acid is added and the flask is stoppered and shaken
- A second flask is made up as a 'blank' containing a drop of sulfuric acid and about 20 cm3 of distilled water
- The reaction is slow, so the reaction mixture is allowed to reach equilibrium over the course of a week
- After a week the flask contents are titrated against 1.0 mol dm-3 sodium hydroxide solution using phenolphthalein as an indicator
- From the difference in the titres of the two flasks, the amount of ethanoic acid at equilibrium can be calculated
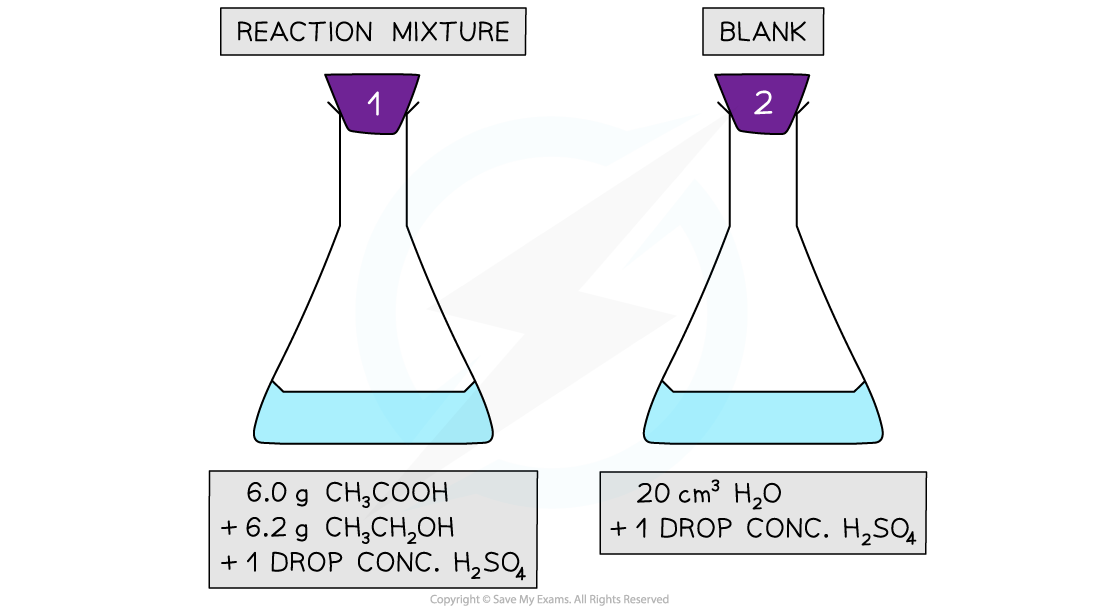
Finding Kc for an esterification
Practical tips
- Although you could measure the liquids by volume, for accurate work it is easier to weigh them directly into the reaction flask and you don't have a problem of drops of reactants remaining in measuring cylinders
- Safety spectacles and gloves should be worn as both acids as very corrosive
- The ethanoic acid has a very strong pungent smell so it is best handled in a fume cupboard
Specimen Results
- Volume of 1.0 mol dm-3 NaOH needed to neutralise the reaction mixture = 32.4 cm3
- Volume of 1.0 mol dm-3 NaOH needed to neutralise the 'blank' flask = 1.4 cm3
Analysis
- Volume of 1.0 mol dm-3 NaOH equivalent to ethanoic acid = 32.4 -1.4 = 31.0 cm3
- Amount of 1.0 mol dm-3 NaOH = (31.0/ 1000) x 1.0 = 0.0031 moles
- The equation for the reaction is
CH3COOH + NaOH → CH3COONa + H2O
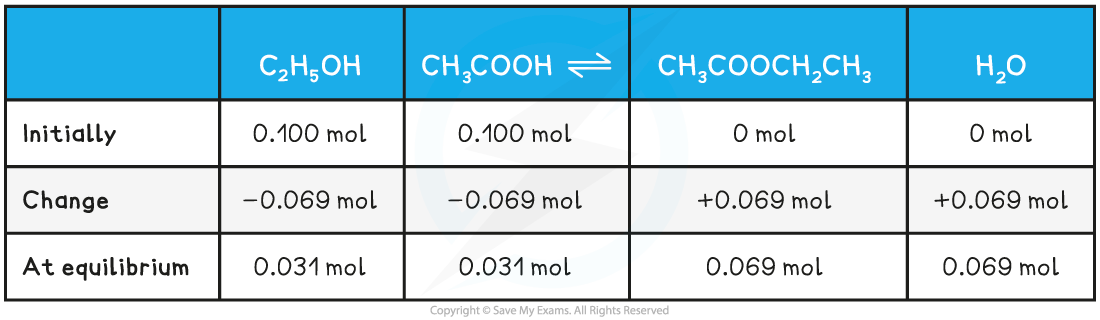
- The moles of ethanoic acid in the equilibrium mixture is = 0.0031 moles
- The equilibrium compositions will be
Equilibrium Composition Table
- The equilibrium constant can be then calculated:
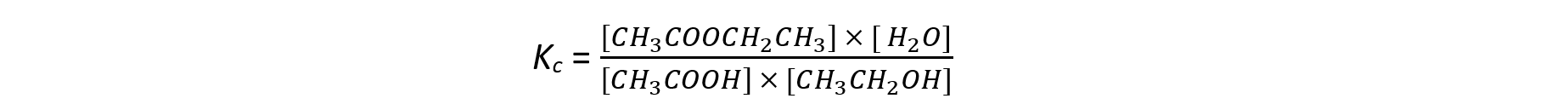
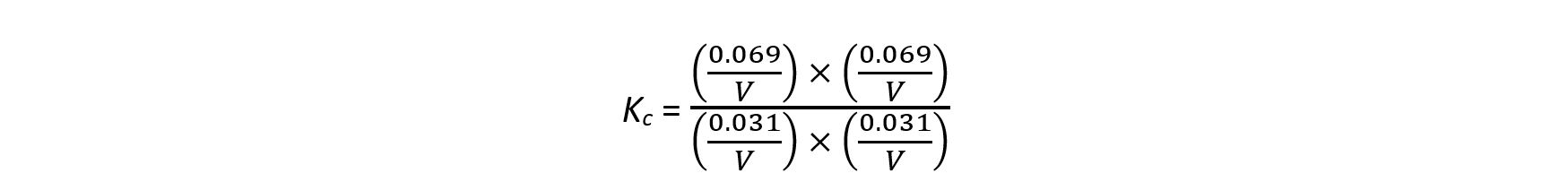
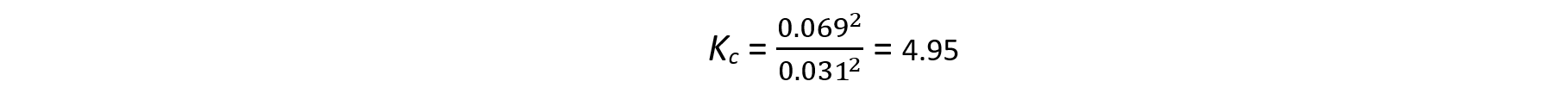
Exam Tip
Note that the equilibrium constant expression uses concentrations rather than moles.In this example, the volume is constant and the concentration terms are the same, so the volumes will cancel out.However, you should always check this because the terms are dependent on the equation and may not always cancel so you will have to calculate the concentrations.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









