- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.1.5 Iodine Clock Reaction
Iodine Clock Reaction
- Clock reactions are so called because they show a sharp dramatic colour change after a period of time has elapsed
- They make ideal reactions for studying kinetics
- Iodine clock reactions come in a number of variations, but they generally all use starch to show a sudden purple-black colour at the end of the reaction
- A common iodine clock reaction uses the reaction between hydrogen peroxide and iodine
H2O2 (aq) + 2I- (aq) + 2H+(aq) → I2 (aq) + 2H2O (l)
- Adding sodium thiosulfate to the reaction mixture uses up the iodine and acts as the reaction timer
2S2O32- (aq) + I2 (aq) → 2I- (aq) + S4O62- (aq)
- The amounts chosen are such that the iodine produced is in excess compared to the other reagents
- Therefore, as soon as the iodine is in excess the blue-black colour of iodine in starch is seen
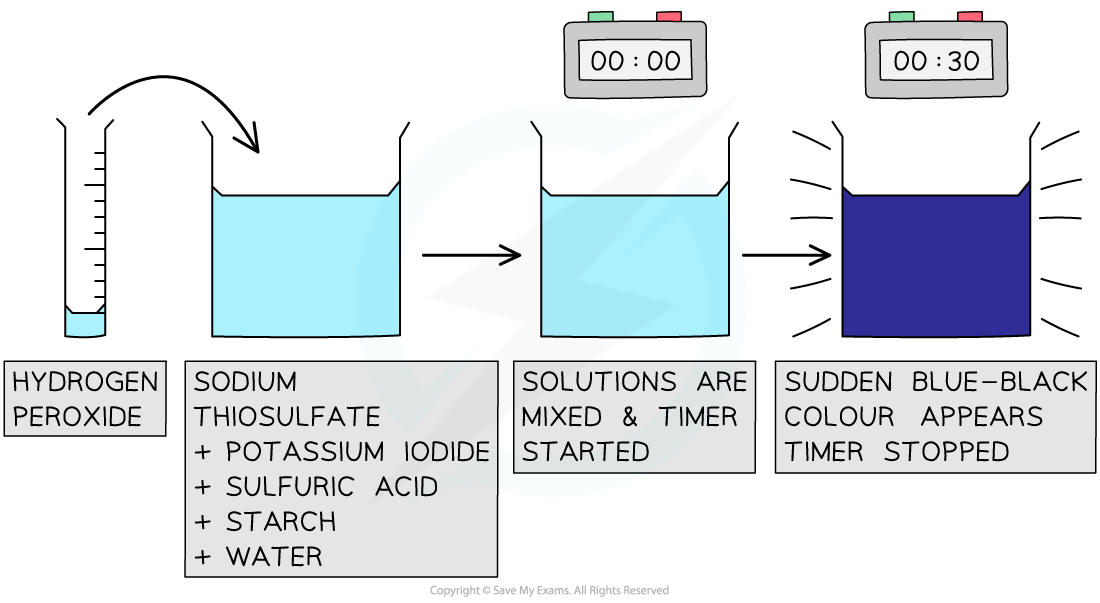
The iodine clock reaction provides a good way to study reaction kinetics
Steps in the procedure
- The solutions are measured in burettes and placed in a small beaker
- The sulfuric acid is in excess so can be measured in a measuring cylinder rather than burette
- The reaction is started by adding 1cm3 of 0.25 mol dm-3 hydrogen peroxide and starting a timer
- The timer is stopped when the blue black colour appears
- Suitable volume compositions to use could be as follows:
Iodine clock volume compositions table
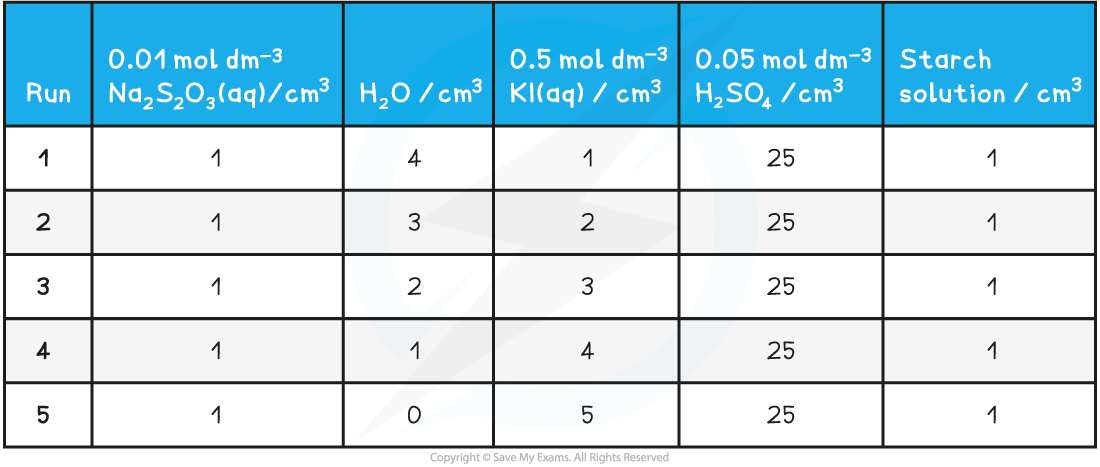
Practical tips
- Hydrogen peroxide is typically found in 'volume' concentrations, based on the volume of oxygen given of when it decomposes:
2H2O2 (aq) → O2 (g) + 2H2O (l)
- For example in school laboratories, a suitable concentration of hydrogen peroxide may be listed as 3% or '10 vol'
- '10 vol' means that when 1cm3 of hydrogen peroxide decomposes it generates 10 cm3 of oxygen
- '10 vol' or 3% hydrogen peroxide has a concentration of 0.979 mol dm3
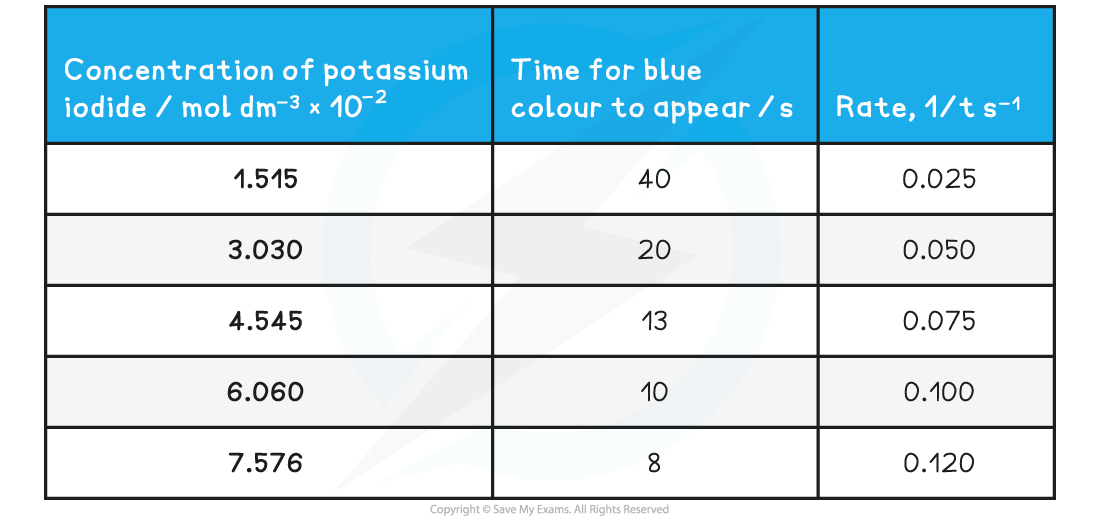
Specimen Results
- Here is a set of typical results for the iodine clock reaction
Specimen results for the iodine clock reaction table
Analysis
- The time of reaction is converted to rate of reaction by calculating the reciprocal value
- A graph is plotted of rate versus concentration
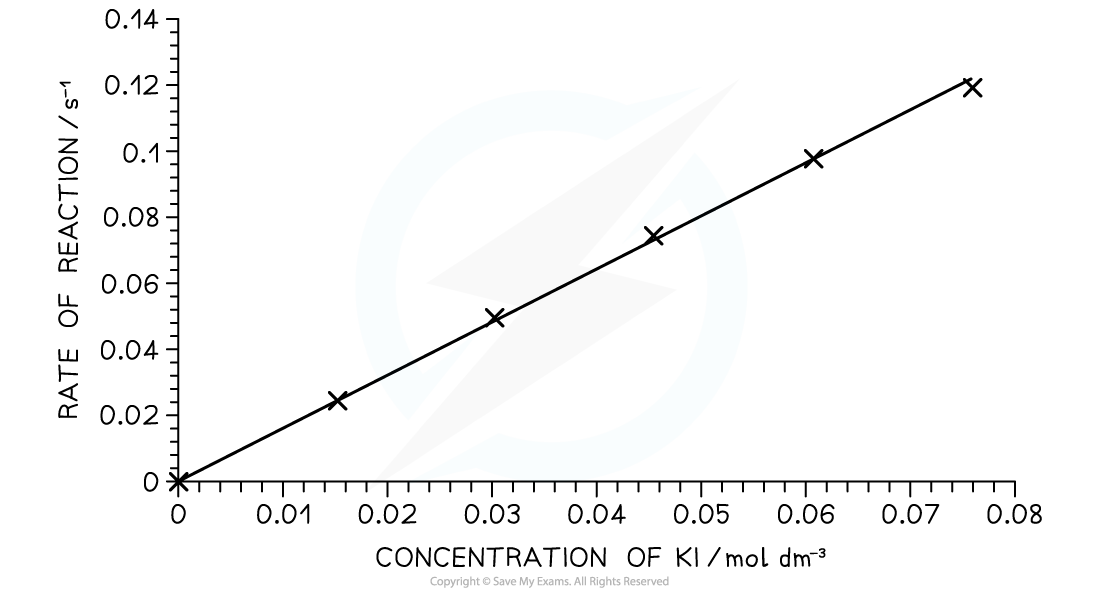
A rate- concentration graph for the iodine clock reaction
- From this graph we can see that the rate of reaction is directly proportional to the concentration of potassium iodide:
- As concentration doubles; the rate of reaction also doubles
- This tells us that the reaction is first order with respect to potassium iodide
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








