- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记8.1.4 Entropy of Vaporisation
Entropy of Vaporisation
- The entropy change when water boils can be measured using a kettle and a top pan balance
- At the boiling point, liquid water and water vapour exist in equilibrium so the free energy change is 0
- Rearranging the Gibbs equation allows us to find the entropy change using the enthalpy change:
ΔGꝋ = ΔHꝋ – TΔSꝋ = 0
ΔHꝋ = TΔSꝋ
ΔSꝋ = ΔHꝋ / T
- The sequence is:
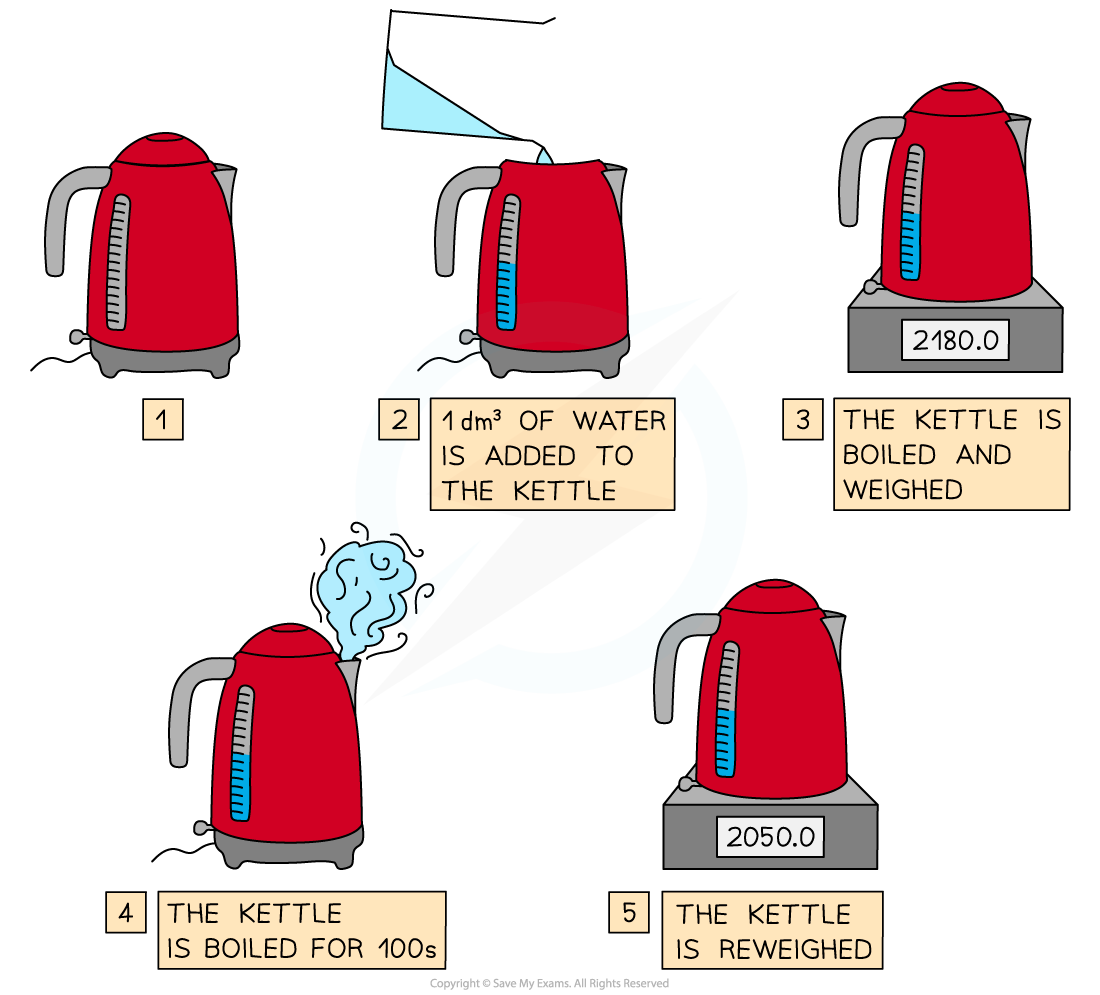
Determining the entropy of vaporisation by measuring the enthalpy change of boiling water
Steps in the procedure
- A fixed volume of water is measured and added to an electric kettle - a suitable amount to use is 1 dm3
- The power rating of the kettle must be known - it will usually be marked somewhere on the kettle
- The water is boiled and then the kettle is switched off and weighed
- The water is re-boiled by keeping the automatic cut-off switch depressed for 100 s
- The kettle and contents are then re-weighed to find out how much water has been lost by evaporation
Practical tips
- Make sure you have a balance that can read up to at least 2.5 kg
- If you don't, you can weigh the water before and after the experiment in a lightweight container such as a plastic box or reduce the volume of water
- The actual amount of water you use does not really matter as long as you can measure it and it covers the heating element in the kettle
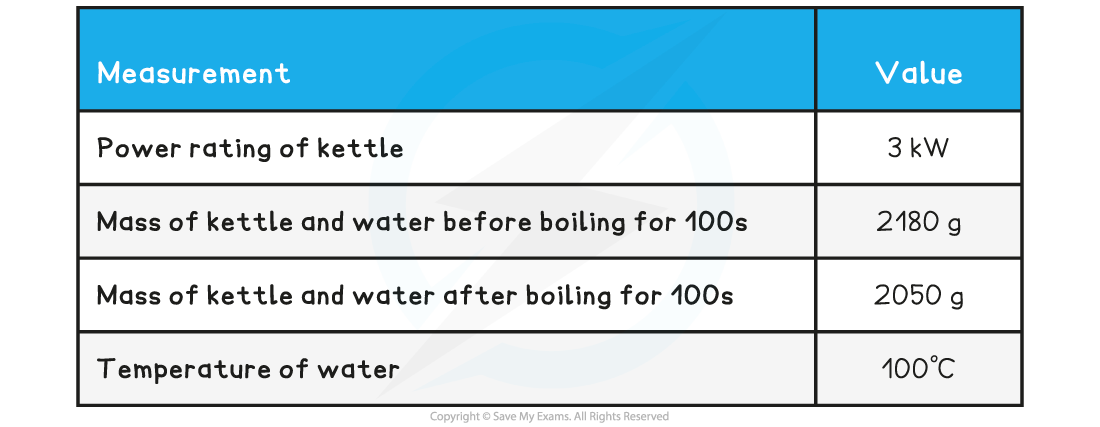
Specimen Results
- Here is a set of typical results for this experiment
Specimen Results Table
Analysis
- Household kettles have a typical power rating of 2 - 3 kW
- Suppose you use a 3 kW kettle for this experiment; this means the kettle supplies 3 kJ of energy per second
- If you boil the kettle for 100 s, then the kettle has supplied 300 kJ of energy to the water
- Using the specimen results we can see that 130 g of water have boiled away, so the number of moles of water that evaporated are:
moles of water evaporated = 130 / 18 = 7.22 mol
- The enthalpy change in kJ per mole of water is therefore:
enthalpy of vaporisation of water = 300 / 7.22 = 41.55 kJ mol-1
- Changing this to J per mole gives:
enthalpy of vaporisation of water = 41.55 x 1000 = 41 550 J mol-1
- The temperature in Kelvin at which water boils is:
temperature in Kelvin = 100 + 273 = 373 K
- Substituting into the equation gives a value for the entropy of vaporisation of water
ΔSꝋ= ΔHꝋ / T
ΔSꝋ= 41 550 / 373 = 111.4 J mol-1
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








