- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.5.4 Nucleophilic Addition–Elimination
Nucleophilic Addition–Elimination
- An important class of reactions that ammonia and amines undergo is nucleophilic substitution with acyl chlorides (or acid chlorides) and acid anhydrides
- These reactions are also called nucleophilic addition-elimination reactions
- Here is a reminder of the functional groups acyl chloride and acid anhydride:

The acyl chloride and acid anhydride group showing the polarity of the functional group
- The delta positive carbon atom is the site of a the nucleophilic attack by the lone pair on the nitrogen in ammonia or the amine
- Chlorine is more electronegative than oxygen, so creates a stronger dipole along the C-Cl bond making the carbon more susceptible to attack from nucleophiles which is which why acyl chlorides are more reactive than acid anhydrides
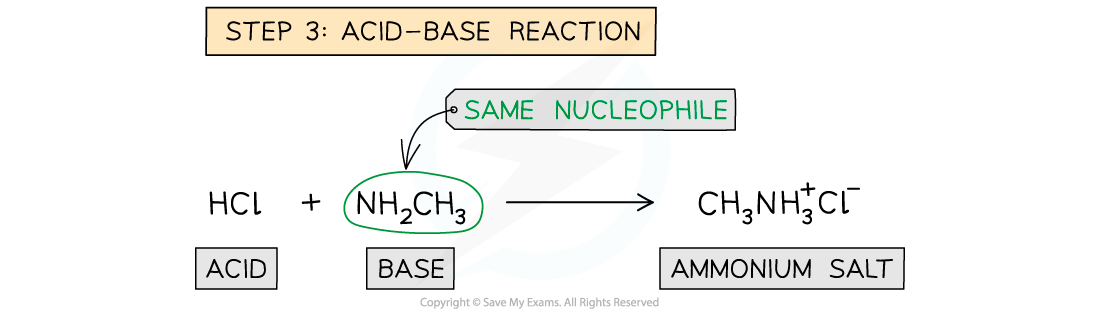
- The reaction produces HCl as an elimination product (hence the name addition-elimination), but since both ammonia and amines are basic the HCl reacts to form ammonium chloride or the amine salt respectively
- Hence, two moles of ammonia/amine are needed in the overall equation:
RCOCl + 2NH3 → RCONH2 + NH4Cl
and
RCOCl + 2R’NH2 → RCONHR’ + R’NH3Cl
Formation of amides: reaction mechanism
- The lone pair on the nitrogen atoms carry out an initial attack on the carbonyl carbon
- This is followed by the elimination of an HCl molecule
- Both reactions of acyl chlorides with ammonia and amines are vigorous however there are also differences
- With ammonia - The product is a non-substituted amide and white fumes of NH4Cl are formed
- With amines - The product is a substituted amide and the HCl formed reacts with the unreacted amine to form a white organic ammonium salt
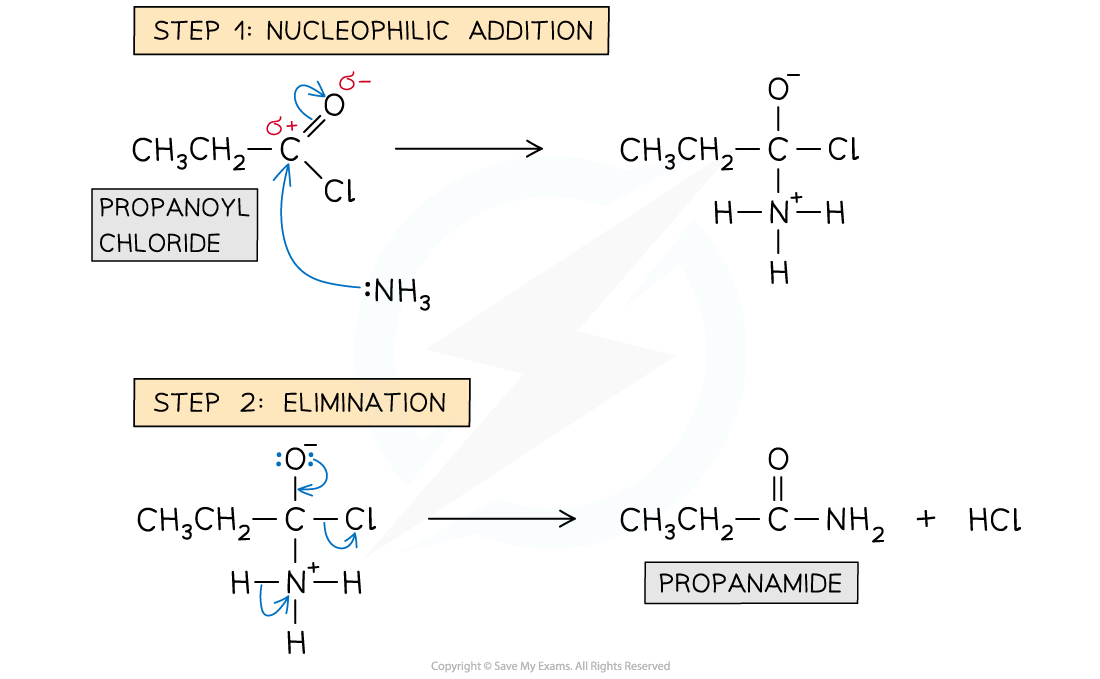
Reaction mechanism of the formation of amides from acyl chlorides with ammonia. The HCl then goes on to react with a molecule of NH3 to form NH4Cl
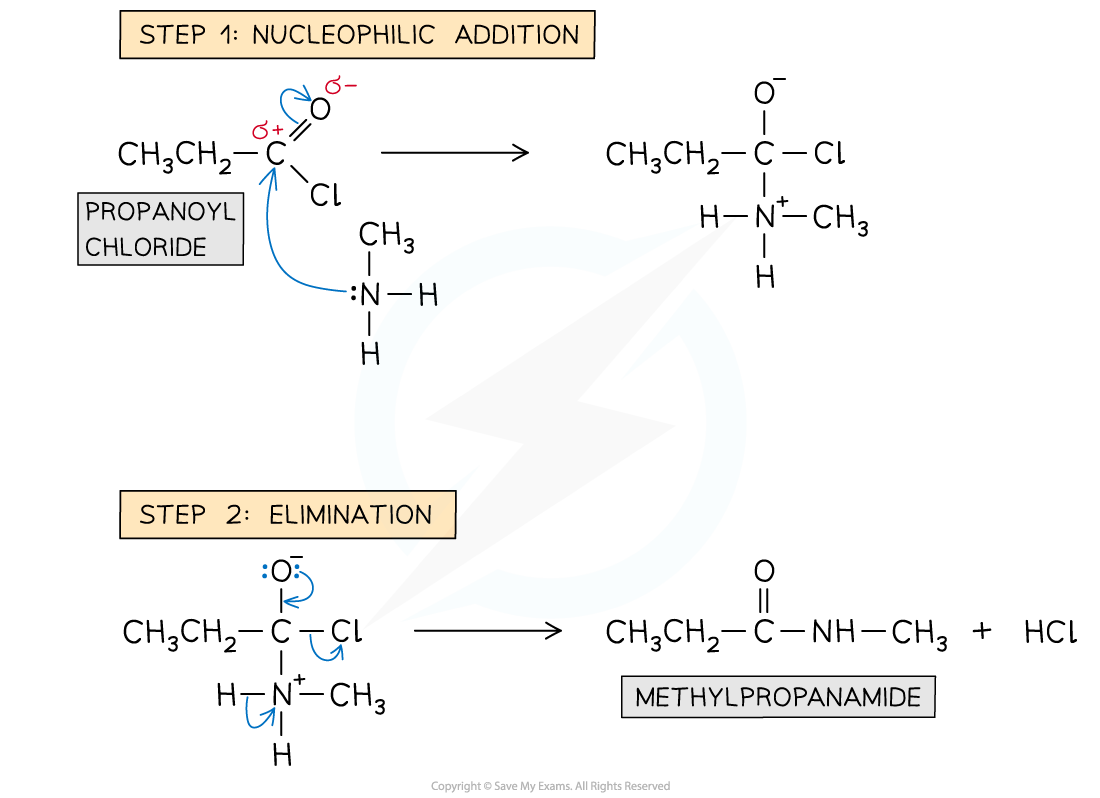
Reaction mechanism of the formation of amides from acyl chlorides with primary amines
Exam Tip
If you want to name substituted amines or amides you can use the prefix letter N, to show that the alkyl (or other group) is attached to the nitrogen, so in the last example calling it N-methylpropanmide avoids any ambiguity about the location of the methyl group.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









