- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.5.3 Nucleophilic Substitution
Nucleophilic Substitution
- The lone pair on the nitrogen atom in amines makes them good nucleophiles, just like ammonia
- When ammonia reacts with a haloalkane a nucleophilic substitution reaction takes place forming a primary amine
- For example chloromethane reacts with ammonia in two steps to make methylamine and ammonium chloride
CH3Cl + NH3 → [CH3NH3]+Cl -
[CH3NH3]+Cl - + NH3 → CH3NH2 + NH4+Cl -
- The methylamine is also a good nucleophile so can undergo further substitution with chloromethane to make dimethylamine
CH3Cl + CH3NH2 → [(CH3)2NH2]+Cl -
[(CH3)2NH2]+Cl - + NH3 → (CH3)2NH + NH4+Cl -
- The dimethylamine can further substitute giving a tertiary amine, trimethylamine
CH3Cl + (CH3)2NH → [(CH3)3NH]+Cl -
[(CH3)3NH]+Cl - + NH3 → (CH3)3N + NH4+Cl -
- The final substitution occurs when the tertiary amine reacts with the chloromethane to make a quaternary ammonium salt
CH3Cl + (CH3)3N → [(CH3)4N]+Cl -
- Since all these multiple substitutions occur it is not a very efficient way to synthesise amines
- If you want to just produce the primary amine then a large excess of ammonia is used to ensure it is the dominant nucleophile in the reaction vessel
Mechanism of nucleophilic substitution
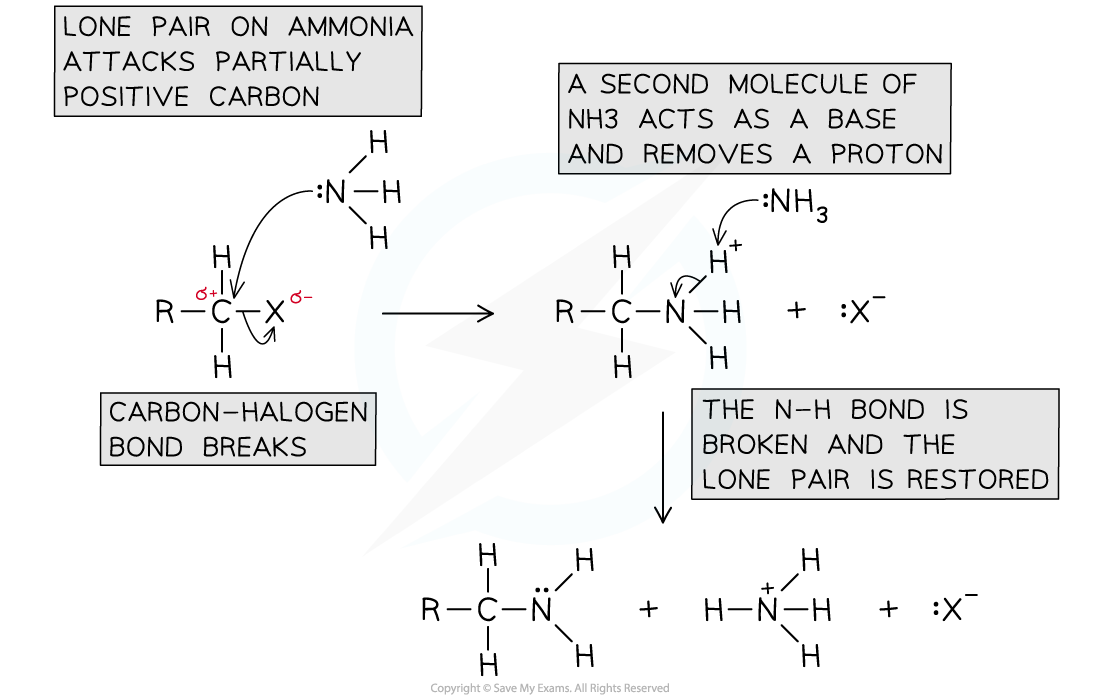
The mechanism of nucleophilic substitution between ammonia and a halogenoalkane
- In the first step the ammonia acts as a nucleophile and in the second step it acts as a base
Exam Tip
Remember that this is the only nucleophilic substitution reaction which needs two moles of the nucleophile. The overall reaction is
RCH2X + 2NH3 → RCH2NH2 + NH4X
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









