- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.3.4 Ester Hydrolysis
Ester Hydrolysis
Hydrolysis of Esters - Acid
- The reverse of the esterification reaction is called hydrolysis
- Ester hydrolysis is a useful reaction for creating biodegradable plastics
- Esters can be hydrolysed to reform the carboxylic acid and alcohol or salts of carboxylic acids by using either dilute acid (e.g. sulfuric acid) or alkali (e.g. sodium hydroxide) and heat
- When an ester is heated under reflux with acid an equilibrium mixture is established, meaning that the hydrolysis reaction is not complete
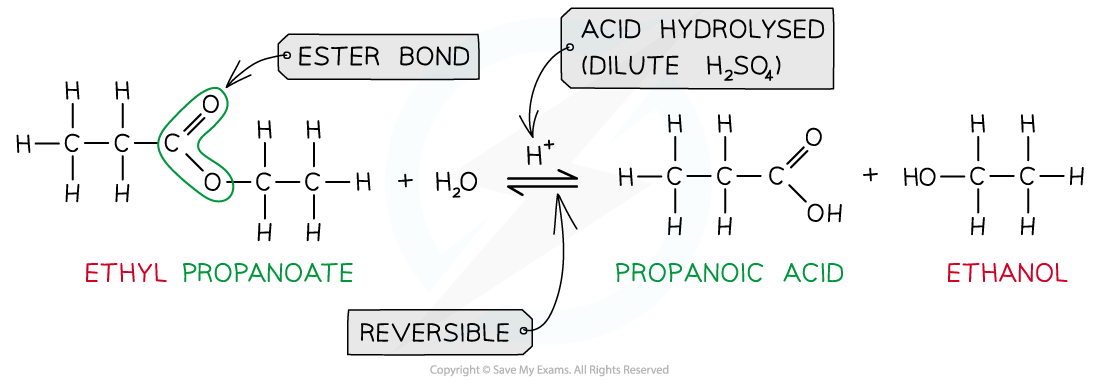
Ester hydrolysis by dilute acid is a reversible reaction forming carboxylic acid and alcohol
Hydrolysis of Esters - Alkaline
- However, heating the ester under reflux with dilute alkali (e.g. sodium hydroxide) is an irreversible reaction as the ester is fully hydrolysed and the reaction goes to completion
- The carboxylic acid produced reacts with excess alkali to form a carboxylate salt and alcohol
- The sodium carboxylate salt requires further acidification to turn into a carboxylic acid
- The sodium carboxylate (-COO-) ion needs to get protonated by an acid (such as HCl) to form the carboxylic acid (-COOH)
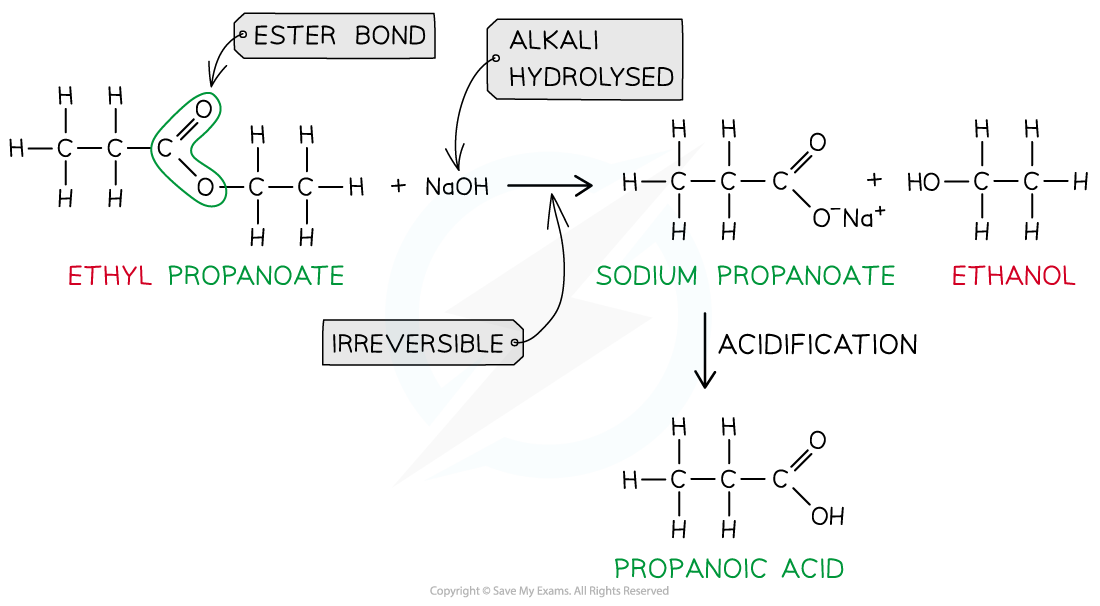
Ester hydrolysis by dilute alkali is an irreversible reaction forming a sodium carboxylate salt and alcohol
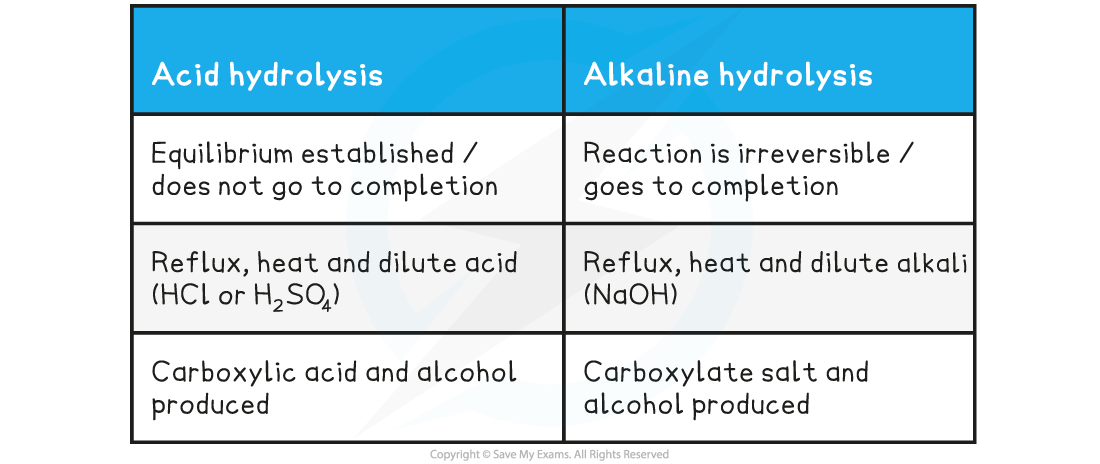
Table showing differences in hydrolysis of esters
Worked Example
Name the products and write equations for the following hydrolysis reaction:
- Ethyl ethanoate with hot dilute sulfuric acid solution
- Methyl propanoate by hot sodium hydroxide solution
Answer:
Answer 1: Ethanoic acid and ethanol
CH3COOCH2CH3 + H2O ⇌ CH3COOH + CH3CH2OH
Answer 2: Sodium propanoate and methanol
CH3CH2COOCH3 + NaOH → CH3CH2COONa + CH3OH
Making Soap
Soaps
- Vegetable oils and animal fats can be hydrolysed in alkaline conditions with aqueous sodium hydroxide to form soaps
- The process is also called saponification
- Soaps are carboxylate salts of long-chain carboxylic acids, known as fatty acids
- When triglycerides / fats are hydrolysed in hot alkaline conditions, the product is a mixture containing glycerol (propane-1,2,3-triol) and the salts of the fatty acids, soaps
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








