- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.3.2 Esters
Esters
Esters
Esters are a carboxylic acid derivative which contains the ester group, -COO-
An ester is named after the parent carboxylic acid from which it is derived
Remove the -oic acid suffix from the parent carboxylic acid and replace with -oate
This part of the name comes from the alcohol, e.g. propanol becomes propylThe alkyl chain attached to the oxygen atom of the -COO- group is then added as the first word in the name
Ester names are confusing because the name is written backwards from the way the structure is drawn

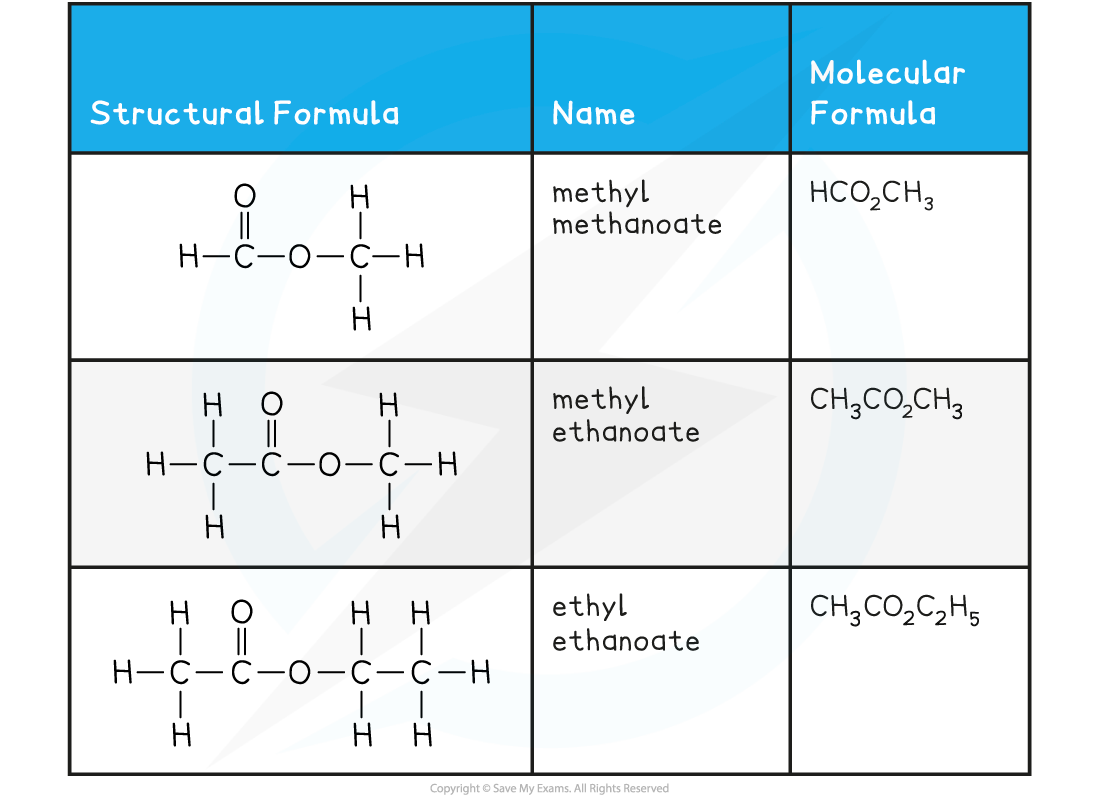
Esters Examples Table
Uses of Esters - fragrances and flavourings
Like aldehydes and ketones, esters are important chemicals in the flavourings and fragrances industry
Esters have a fruitier and sweeter smell than aldehydes and ketones and are responsible for many natural flower scents and flavours
Synthetic esters can also be used as artificial fruit flavours in confectionary products e.g. ethyl methanoate is used as a raspberry ester fragrance
Aromas and tastes are often due to complex mixtures of different esters
Testing for alcohols
The sweet and fruity smell of an ester can be used as a test for the presence of an alcohol or carboxylic acid
An unknown substance can be warmed with a carboxylic acid such as ethanoic acid in the presence of concentrated sulfuric acid (catalyst)
Excess acid with its pungent vinegary smell can be removed by adding warm aqueous sodium carbonate solution
If the remaining mixture has a sweet smell of an ester, this confirms the presence of an alcohol
The warmth of the solution then causes the ester to evaporate and the sweet smell is easily detected
Uses of Esters - plasticisers
Poly(chloroethene) better known as PVC, is a strong rigid polymer suitable for making drainpipes and guttering
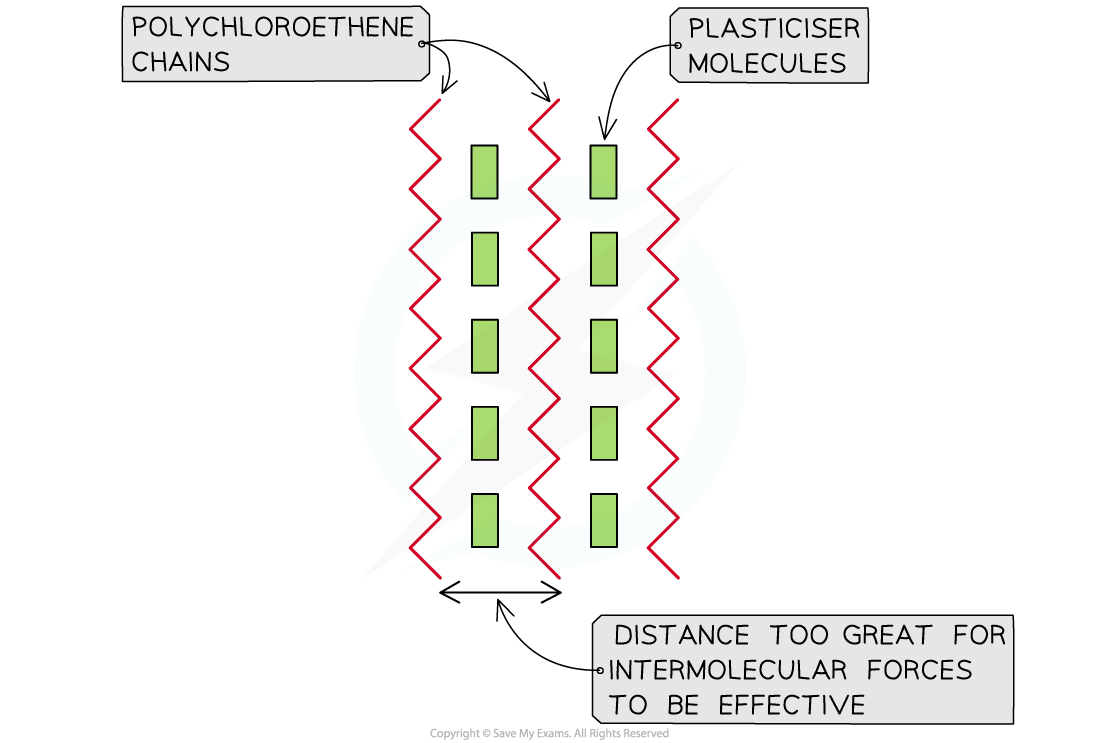
When a suitable plasticising ester is added, it can be made into cling film which is soft and pliable
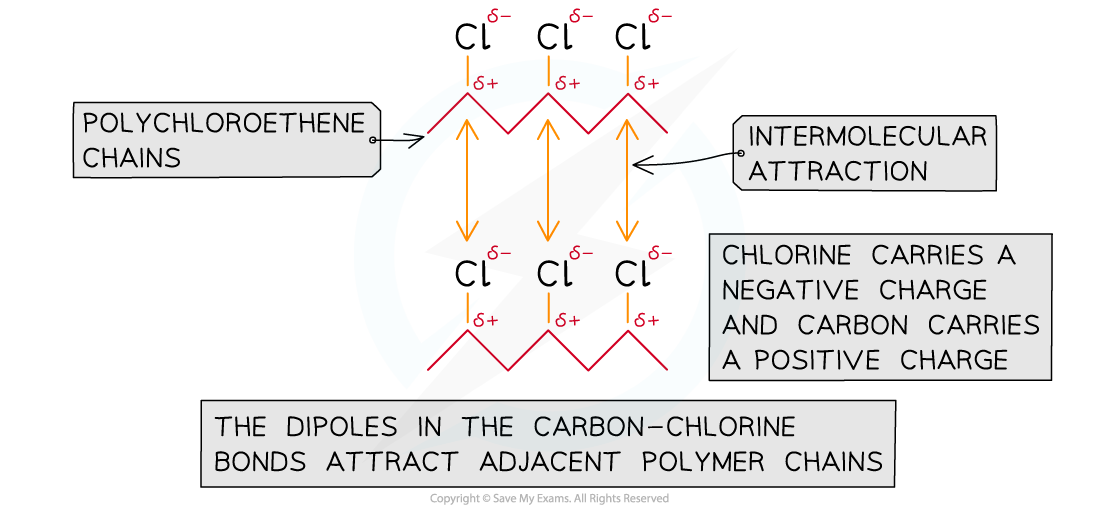
An ester plasticiser works by penetrating between the polymer chains and increasing the distance between them
This then weakens the polar effects of the carbon-chlorine bond and the rigidity of the 3D structure if reduced allowing the polymer chains to slide over one anotherEsters can also be used as plasticisers, which are additives mixed into polymers to increase the flexibility of the polymer
Intermolecular forces in PVC
Addition of a plasticiser reduced rigidity in PVC
Uses of Esters - solvents
Ethyl ethanoate is a common solvent which has the beneficial properties of low toxicity and low volatility (its boiling point is 77˚C) as well as being relatively cheap
This makes it an ideal solvent for use in glues, fragrances and nail varnishesEsters are also commonly used as solvents for organic compounds
Naturally occurring Esters
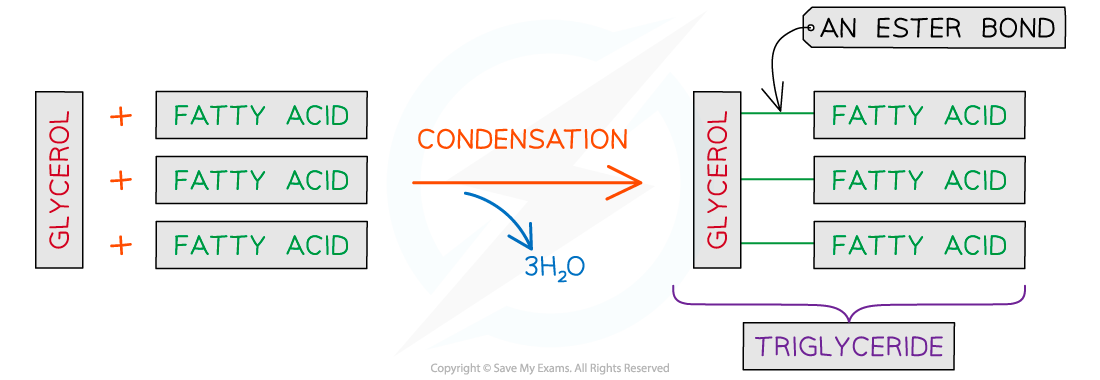
Triglycerides found in animal fat and olive oil are naturally occurring esters
They are tri-esters of glycerol (propane-1,2,3-triol) and fatty acids (long chain carboxylic acids) such as stearic acid (CH3(CH2)16COOH)
The reaction of 1 mole of glycerol with 3 moles of a fatty acid in the presence of an acid catalyst leads to the formation of the triglyceride whereby 3 moles of water are eliminated
The synthesis of a triglyceride from glycerol and fatty acid chains
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








