- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.2.3 Nucleophilic Addition
Nucleophilic Addition
- Many of the reactions which carbonyl compounds undergo are nucleophilic addition reactions
- The carbonyl group -C=O, in aldehydes and ketones is polarised
- The oxygen atom is more electronegative than carbon drawing electron density towards itself
- This leaves the carbon atom slightly positively charged and the oxygen atom slightly negatively charged
- The carbonyl carbon is therefore susceptible to attack by a nucleophile, such as the cyanide ion
The carbonyl group here has a dipole with a delta positive carbon and a delta negative oxygen

General Mechanism with an aldehyde: General Mechanism with a ketone:
General Mechanism with a ketone: 
In both reactions, the nucleophile (Nu) attacks the carbonyl carbon to form a negatively charged intermediate which quickly reacts with a proton
Addition of HCN to carbonyl compounds
- The nucleophilic addition of hydrogen cyanide to carbonyl compounds is a two-step process, as shown below

- In step 1, the cyanide ion attacks the carbonyl carbon to form a negatively charged intermediate
- In step 2, the negatively charged oxygen atom in the reactive intermediate quickly reacts with aqueous H+ (either from HCN, water or dilute acid) to form 2-hydroxynitrile compounds,
- e.g. 2-hydroxypropanenitrile
Exam Tip
By convention, we write the formula of an ion then its charge, e.g. CN-.
- The actual negative charge on the cyanide ion is on the carbon atom and not on the nitrogen atom.
- However, when writing it together as :CN- you will not be penalised for writing the minus charge after the N.
- This reaction is important in organic synthesis, because it adds a carbon atom to the chain, increasing the chain length
- The products of the reaction are hydroxynitriles
- The nitrile group is the priority functional group so it is attached to carbon 1 and results in the suffix -nitrile
- The hydroxyl group is not the priority functional group so the hydroxyl group is named using the hydroxy- prefix, rather than the -ol suffix
Forming Enantiomers
Forming Enantiomers
- Even if a starting material does not display optical isomerism, it can still form a product which does display optical isomerism
- This is the case when aldehydes and ketones undergo nucleophilic addition with hydrogen cyanide, HCN
- Due to the shape of the aldehyde or ketone, the :CN- can attack on either side of the carbonyl
- When it attacks on one side, it will produce one enantiomer and when it attacks on the other side, it will produce the other enantiomer
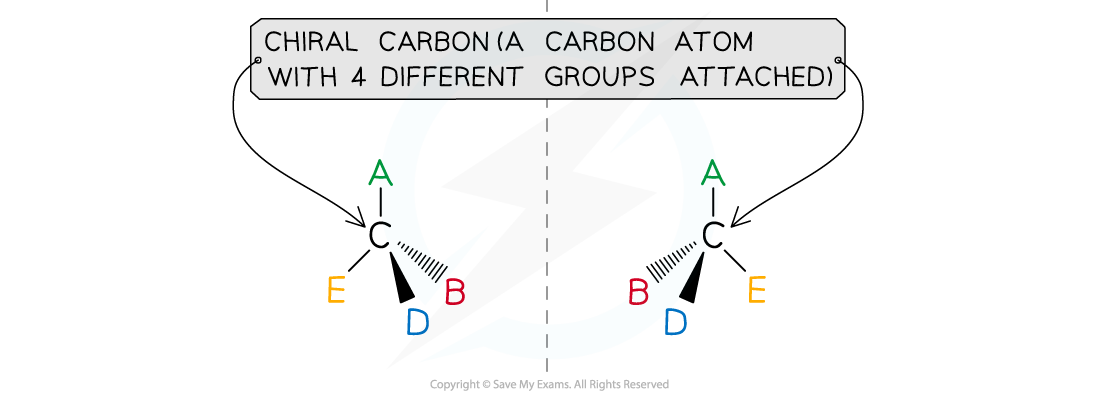
- When it attacks on one side, it will produce one enantiomer and when it attacks on the other side, it will produce the other enantiomer
- The reaction mixture which is produced will be a racemic mixture
- There will be a 50:50 mixture of both enantiomers, because there is a 50:50 chance of attack happening on each side
- Racemic mixtures are formed when addition reactions are done with a planar starting material, because the reaction takes place with equal probability from either side of the plane

The attack from the :CN- has a 50:50 chance of taking place on either side of the C=O bond
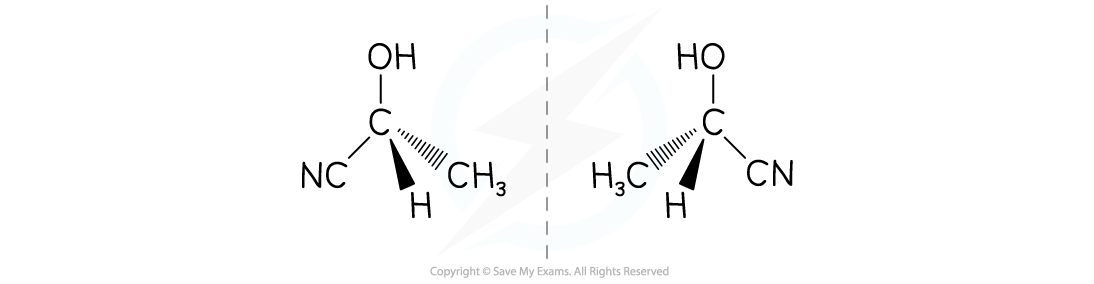
A racemic mixture, or racemate, of each enantiomer is formed
- The enantiomers in a racemic mixture both rotate plane polarised light, but they rotate it in opposite directions
- Because there is a 50:50 mixture of both enantiomers, each rotating light in equal amounts but opposite directions, the effects on plane polarised light are cancelled out
- Therefore, there will be no effect on plane polarised light with a racemic mixture
- The optical rotation of the racemic mixture is zero
- This can be used as a test to determine whether a mixture is racemic
- If you know that a sample contains enantiomers of chiral compounds, and when tested there is no effect on plane polarised light, then the reaction mixture must be racemic
- If there is an effect on plane polarised light, then the sample is not racemic
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









