- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记7.2.2 Reduction of Carbonyls
Reduction of Carbonyls
Reduction of Carbonyls
- There are a large number of reducing agents which will reduce both an aldehyde and a ketone to an alcohol
- Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohols
- Possibly the most common reducing agent for this is sodium tetrahydridoborate, NaBH4
- You may also see this named as sodium borohydride in some sources
- In an aqueous solution, NaBH4 generates the hydride ion nucleophile, :H-
- The hydride ion will reduce a carbonyl group in an aldehyde or a ketone, but is not strong enough to reduce a C=C double bond
- This is because it is attracted to the C in the C=O bond, but is repelled by the high electron density of the C=C bond
- When this reaction takes place, it is an example of a nucleophilic addition reaction
Reduction Reactions
- Carboxylic acid to a primary alcohol:
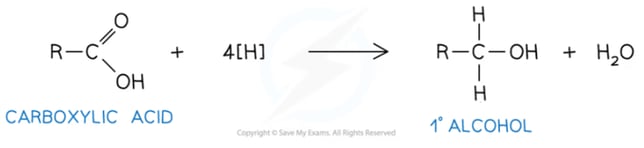
- Aldehyde to a primary alcohol:

- Ketone to a secondary alcohol:

Exam Tip
In theory the reduction of a carboxylic acid is a two stage process, from the carboxylic acid to the aldehyde and then further reduction from the aldehyde to the primary alcohol. In reality however, the reaction would really go from the carboxylic acid straight to the primary alcohol. Be careful and check the wording of the question when asked about the reduction of a carboxylic acid!
Exam Tip
Lithium tetrahydridoaluminate, LiAlH4, in a non-aqueous solvent can also be used as a reducing agent for the reduction of carbonyls.
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









