- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记 6.1.4 Oxides Reacting with Water
Oxides Reacting with Water
Structure, bonding & electronegativity of the Period 3 elements table
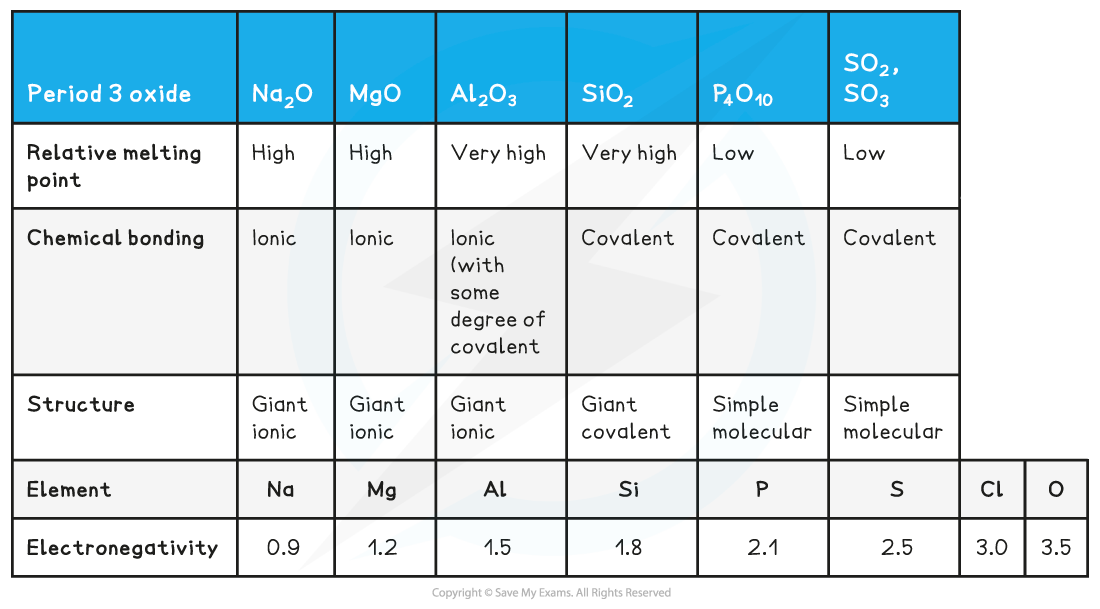
- The oxides of Na and Mg which show purely ionic bonding produce alkaline solutions with water as their oxide ions (O2-) become hydroxide ions (OH-):
O2- (aq) + H2O (l) → 2OH- (aq)
- The oxides of P and S which show purely covalent bonding produce acidic solutions with water because when these oxides react with water, they form an acid which donates H+ ions to water
- Eg. SO3 reacts with water as follows:
SO3 (g) + H2O (l) → H2SO4 (aq)
-
- The H2SO4 is an acid which will donate a H+ to water:
H2SO4 (aq) + H2O (l) → H3O+ (aq) + HSO4- (aq)
- Al and Si are insoluble and when they react with hot, concentrated alkaline solution they act as a base and form a salt
- This behaviour is very typical of a covalently bonded oxide
- Al can also react with acidic solutions to form a salt and water
- This behaviour is very typical of an ionic bonded metal oxide
- This behaviour of Al proves that the chemical bonding in aluminium oxide is not purely ionic nor covalent: therefore it exhibits amphoteric character
Reaction of Period 3 oxides with water table
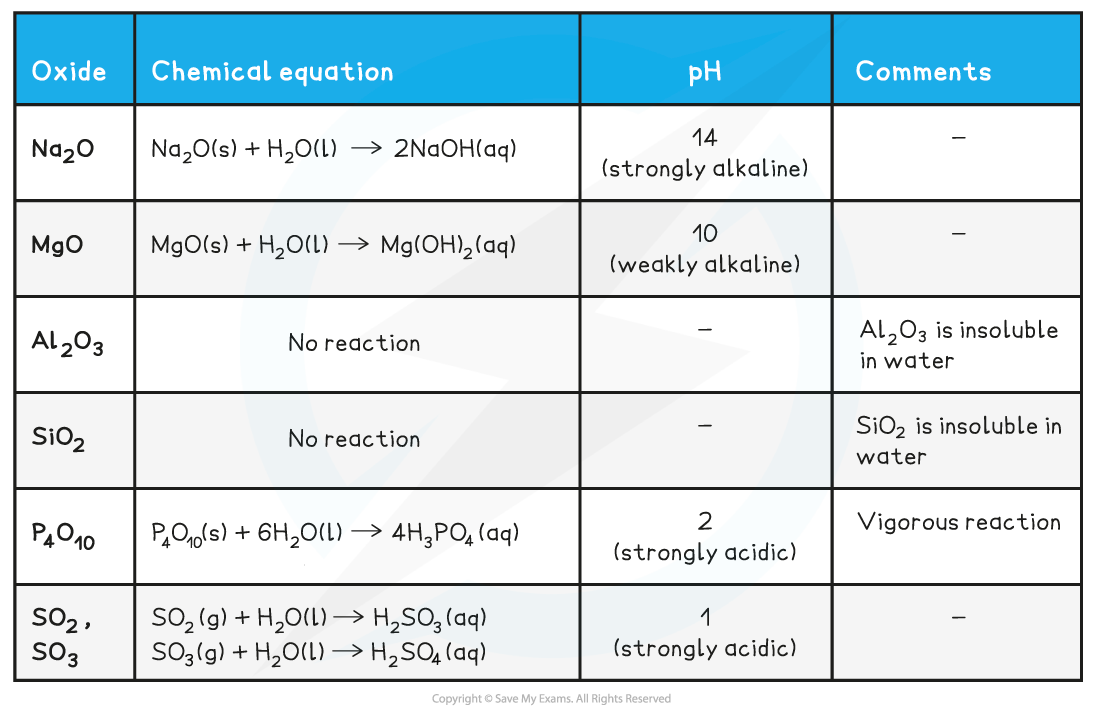
Behaviour of the Period 3 Oxides with Water
- Metal oxides (to the left of the periodic table):
- Sodium oxide, Na2O, and magnesium oxide, MgO, are made up of ions
- They contain an oxide ion, O2-, which is a strong base and will readily produce hydroxide ions through reaction with water
- This is why the solutions formed are strongly alkaline
- Sodium oxide forms a more alkaline solution than magnesium oxide because it is far more soluble in water
- Oxides in the middle of the periodic table
- Although ionic, aluminium oxide does not react with water because the oxide ions are held too strongly in the ionic lattice
- This means the ions cannot be separated
- Silicon dioxide is a giant covalent molecule - it is the main component of sand
- It has millions of strong covalent bonds, so it does not react with water
- Non-metal oxides (to the right of the periodic table):
- Oxides of phosphorus and sulfur are simple covalent molecules
- They will react with water to produce acidic solutions
Exam Tip
Key thing to remember: The metal oxides form alkaline solutions in water, the oxides in the middle do not react and the non-metal oxides form acidic solutions.
Acid-Base Reactions of the Oxides
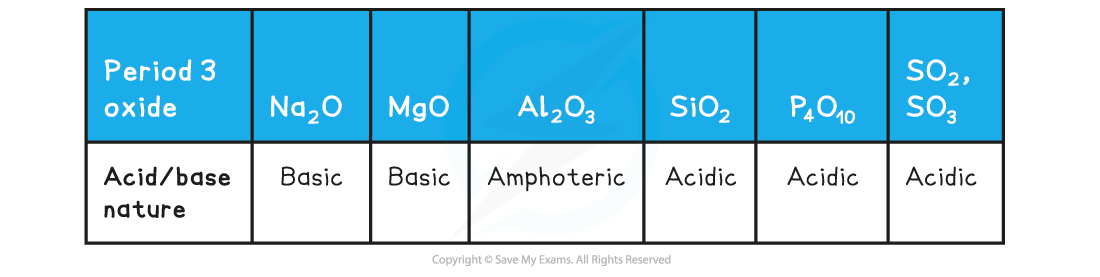
Acid/base Nature of the Period 3 Oxides
- Aluminium oxide is amphoteric which means that it can act both as a base (and react with an acid such as HCl) and an acid (and react with a base such as NaOH)
Reactions of the Period 3 oxides with acid/base table
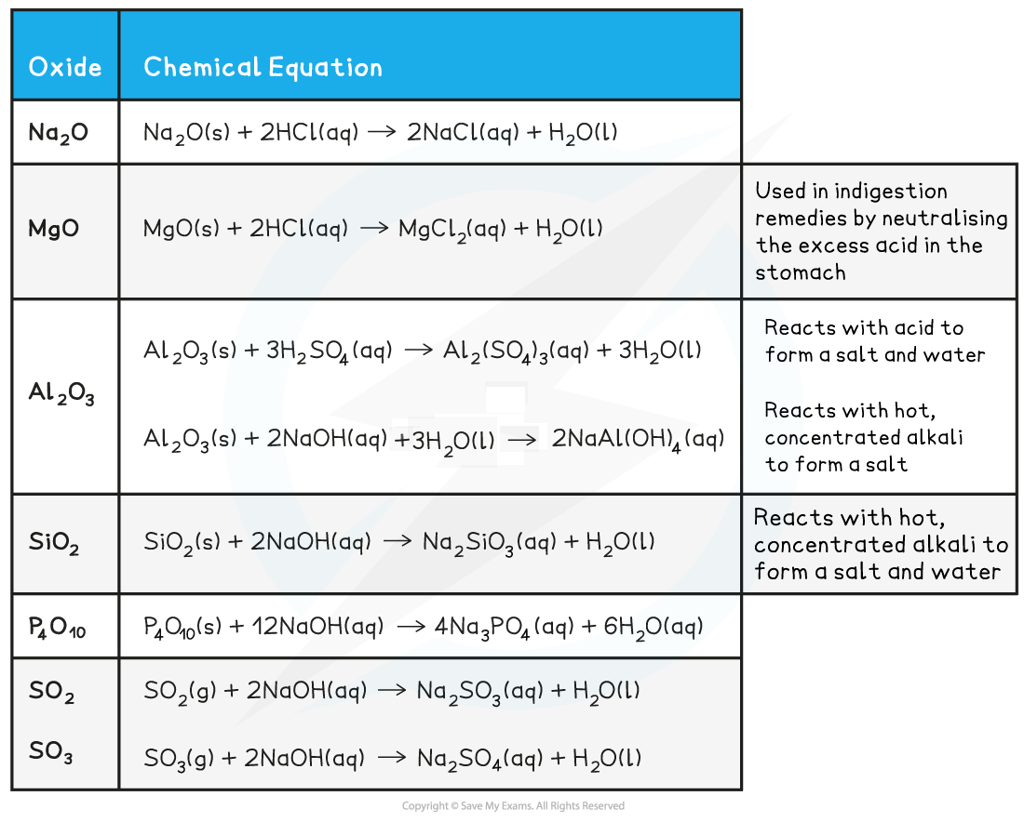
Exam Tip
It is crucial that you learn these reactions - make sure that you know the state symbols, the products formed and the full balanced equations!
转载自savemyexams

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1









