- 翰林提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
AQA A Level Chemistry复习笔记5.6.3 Titrations
Titrations
- Volumetric analysis is a process that uses the volume and concentration of one chemical reactant (a standard solution) to determine the concentration of another unknown solution
- The technique most commonly used is a titration
- The volumes are measured using two precise pieces of equipment, a volumetric or graduated pipette and a burette
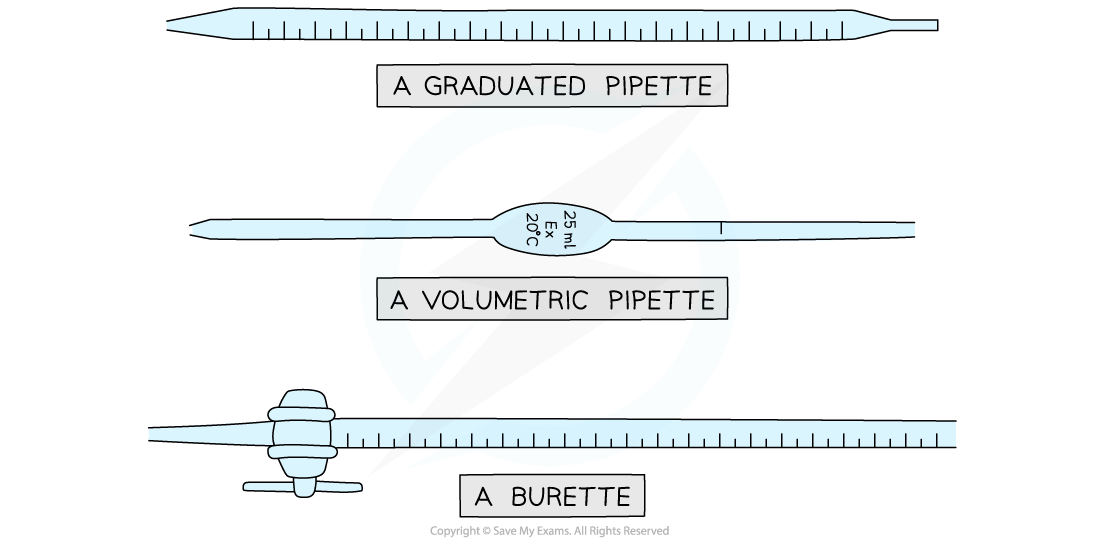
Equipment used to measure volumes precisely in titrations
- Burettes are usually marked to a precision of 0.10 cm3
- Since they are analogue instruments, the uncertainty is recorded to half the smallest marking, in other words to ±0.05 cm3
- The stoichiometric point or equivalence point occurs when the two solutions have reacted completely and is shown with the use of an indicator
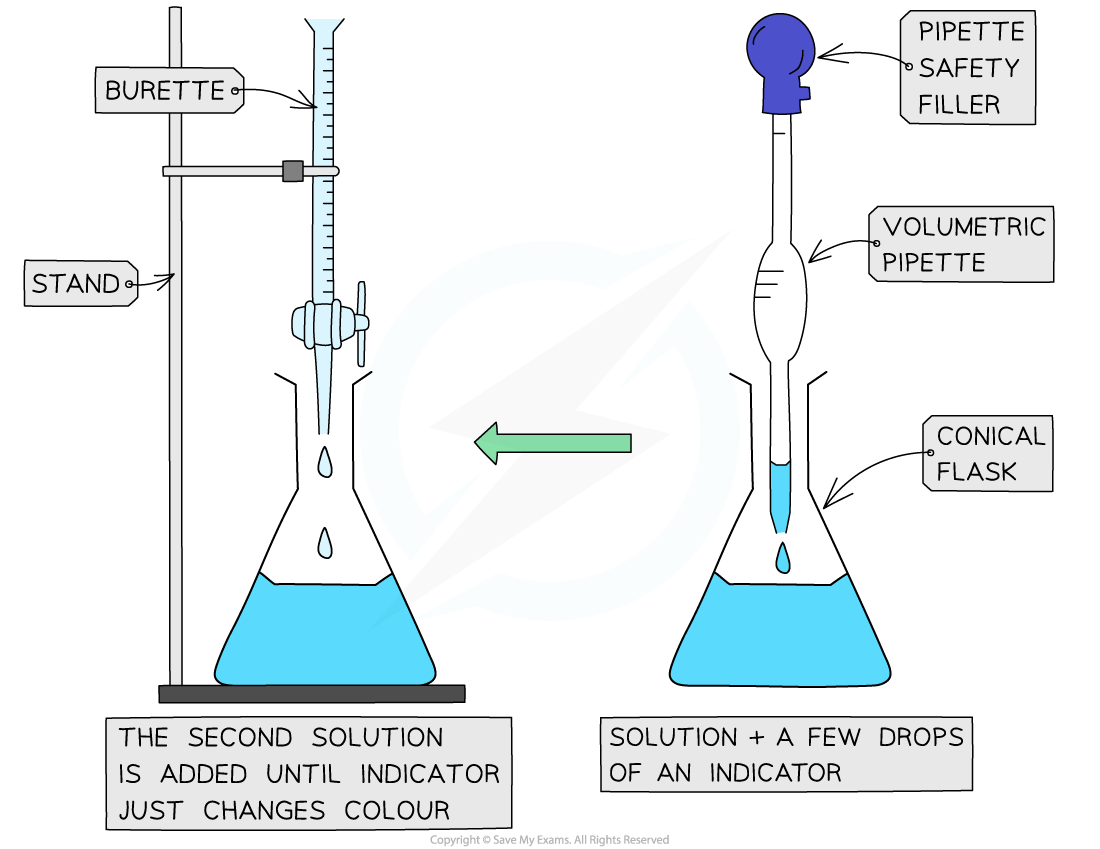
The steps in a titration
- The steps in a titration are:
- Measuring a known volume (usually 20 or 25 cm3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask
- The other solution is placed in the burette
- A few drops of the indicator are added
- The tap on the burette is carefully opened and the solution added, portion by portion, to the conical flask until the indicator just changes colour(this is the end point)
- Multiple trials are carried out until concordant results are obtained
Titration Calculations
- Titration calculations are used to find the concentration of unknown solutions
- They can also be used to calculate the pH after a given point during a titration
Worked Example
Example 1: Calculations from titration resultsIn a titration, 25.00 cm3 of 0.05 mol dm-3 hydrochloric acid was neutralised by 8.50 cm3 of sodium hydroxide solution. Calculate the concentration of the sodium hydroxide solution.
Answer
Step 1: Find the number of moles of acid
moles of acid = concentration x volume in dm3
moles of acid = 0.05 x 25/1000 = 1.25 x 10-3 mol
Step 2: Deduce the number of moles of alkali
The equation for the reaction shows the mole ratio is 1:1
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
∴ moles of alkali = 1.25 x 10-3 mol
Step 3: Work out the concentration of the alkali
concentration = moles/volume in dm3
concentration = 1.25 x 10-3/0.0085 = 0.15 mol dm-3
Worked Example
Example 2: Calculating the pH in a strong acid-strong base titration50.0 cm3 of 0.10 mol dm3 NaOH is gradually added to 25.0 cm3 of 0.15 mol dm3 hydrochloric acid. Determine the pH after 45 cm3 of NaOH has been added. (Kw = 1 x 10-14 mol2 dm-6 at 298 K)
Answer
Step 1: Find the number of moles of acid
moles of acid = concentration x volume in dm3
moles of acid = 0.15 x 25/1000 = 3.75 x 10-3 mol
Step 2: Deduce the number of moles of alkali added
The equation for the reaction shows the mole ratio is 1:1
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
moles of alkali added = 0.10 x 45/1000 = 4.50 x 10-3 mol
∴ moles of alkali in excess = (4.50 x 10-3- 3.75 x 10-3) = 7.5 x 10-4 mol
Step 3: Work out the concentration of the alkali
concentration = moles/volume in dm3
concentration = 7.5 x 10-4/0.070 = 0.0107 mol dm-3
Step 4: Use Kw to find the concentration of H+
Kw = [H+][OH-]
[H+] = Kw /[OH-] = 1.00 x 10-14/0.0107 = 9.35 x 10-13
Step 5: Find the pH
-log[H+] = -log(9.35 x 10-13)
pH = 12.03
Exam Tip
You will be asked to perform calculations only on monoprotic acids. A monoprotic acid has only one acidic hydrogen, like HCl. Sulfuric acid, H2SO4, is a diprotic acid.
转载自savemyexams

早鸟钜惠!翰林2025暑期班课上线

最新发布
© 2025. All Rights Reserved. 沪ICP备2023009024号-1








